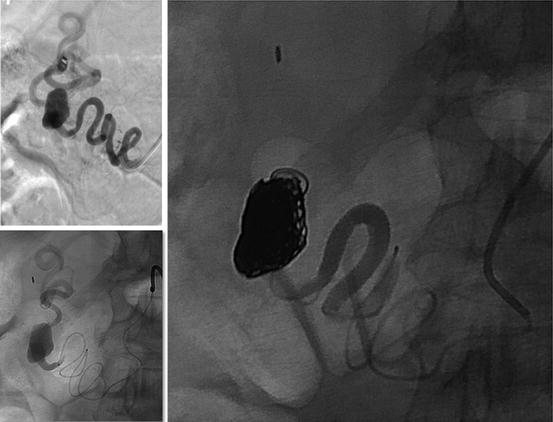
Fig. 34.1
Multiple intrahepatic aneurysms in a patient with polyarteritis nodosa treated by coil embolization and corticosteroid therapy
Superior Mesenteric Artery Aneurysms (SMAAs)
Non-ruptured SMAAs are more likely to cause symptoms compared with other VAAs; in addition to abdominal pain, patients may present with nausea, vomiting, gastrointestinal bleeding, or weight loss (Fig. 34.2). The high mortality rate is due to a free rupture into the peritoneal cavity with accompanying intestinal ischemia [6].


Fig. 34.2
Autopsy specimen with multiple small superior mesenteric artery branch aneurysms in a patient with indeterminate connective tissue disorder who died of ruptured visceral artery aneurysm
Celiac Artery Aneurysms (CAAs)
Most CAAs are symptomatic, presenting with vague abdominal pain. Patients experiencing biliary obstruction may also present with gastrointestinal bleeding and jaundice. The “double rupture” phenomenon seen in SAAs is reported in nearly 25 % of ruptured CAAs [6].
Gastric Artery Aneurysms (GAAs) and Gastroepiploic Artery Aneurysms (GEAAs)
More than 90 % of reported GEAAs are ruptured on initial presentation, resulting in hemorrhage into the peritoneum. They usually present with mild epigastric pain, hemoperitoneum, and hemorrhagic shock requiring some form of intervention [6].
Pancreaticoduodenal Artery Aneurysms (PDAAs) and Gastroduodenal Artery Aneurysms (GDAAs)
Symptoms arising from PDAAs and GDAAs are most often vague and include epigastric abdominal pain that may radiate to the back (Fig. 34.3). Other symptoms may include gastrointestinal bleeding, hypotension, emesis, diarrhea, and jaundice. Of 74 GDAAs reported between 1956 and 2011, a gastrointestinal hemorrhage secondary to rupture of the aneurysm was the most common clinical presentation (52 %), followed by abdominal pain (46 %); only 7.5 % of the patients were asymptomatic. Rupture of a PDAA can cause potentially fatal bleeding into the retroperitoneal space, abdominal cavity, or gastrointestinal tract [6, 8].
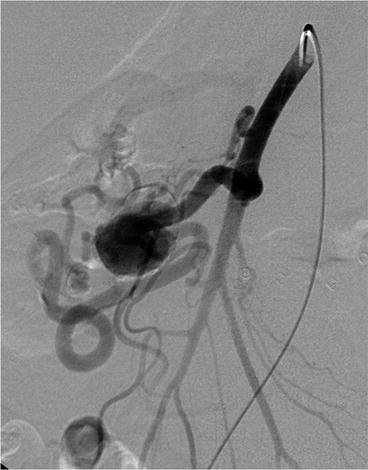

Fig. 34.3
Large gastroduodenal branch artery aneurysm
Inferior Mesenteric, Jejunal, Ileal, and Colic Artery Aneurysms
Although abdominal pain and hypovolemic shock are seen in approximately 85 % of the cases [6, 9], around 50 % of inferior mesenteric artery aneurysms (IMAAs) are asymptomatic. On reviewing 54 IMAAs, 21 were associated with SMA (Fig. 34.4) and CA occlusion [10]. For middle colic artery aneurysms, patients usually present with abdominal pain, vomiting, and a sudden unexpected drop in hemoglobin [11].
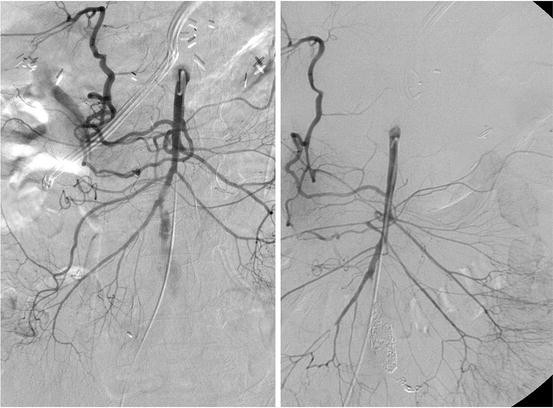

Fig. 34.4
SMA branch aneurysm treated by coil embolization
Epidemiology and Etiology
The etiology of VAAs differs for true aneurysms versus pseudoaneurysms. Causes of true VAAs include atherosclerosis, medial degeneration, collagen vascular diseases, and fibromuscular dysplasia (FMD). Other factors such as multiparity, portal hypertension, and posttransplant status have been associated specifically with VAA formation. Of 927 liver transplant recipients, 21 developed VAAs (2.3 %); the majority involved hepatic and splenic arteries [12]. Rare medical conditions, such as von Recklinghausen’s disease, type VI Ehlers-Danlos syndrome (EDS), and polyarteritis nodosa (PAN), have been implicated in the development of VAAs. Reported case series have demonstrated the association of multiple VAAs with systemic arteritis, endocarditis with septic emboli, connective tissue disorders, and even excessive acetaminophen use [6].
Visceral pseudoaneurysms, defined as a tear in the vessel wall with resultant peri-artery hematoma, seem to be less frequent than true aneurysms and can be caused by trauma, iatrogenic interventions (from surgical, laparoscopic, or interventional treatments), inflammatory conditions (pancreatitis), or infectious disease (mycotic aneurysm) [13].
Splenic Artery Aneurysms (SAAs)
The incidence of SAAs in the general population based on autopsy studies and angiography series has been estimated between 0.01 % and 0.78 %, and 10 % in an elderly population autopsy series.
Most SAAs are related to atherosclerosis, arterial fibrodysplasia, and arteritis; most patients are young. Associated risk factors include female gender, with a ratio of 4:1 over male, multiparity, and portal hypertension. The association between SAA and portal hypertension has been documented, especially in patients undergoing orthotopic liver transplantation. Eleven evaluated patients with known portal hypertension or cirrhosis showed a likely SAA incidence ranging from 10 % to 20 %, 114 with one study reporting an incidence as high as 50 % [6].
Pseudoaneurysm formation associated with pancreatitis has been attributed to digestion of the splenic artery by pancreatic enzymes. Splenic artery pseudoaneurysm formation associated with chronic pancreatitis represents the leading cause of hemosuccus pancreatitis and is a challenging clinical scenario [6].
Hepatic Artery Aneurysms (HAAs)
The incidence of HAAs is estimated to be less than 0.4 % in the general population, and with increasing use of hepatobiliary instrumentation and manipulation, the incidence appears to increase. Hepatic artery pseudoaneurysm formation after liver transplantation is estimated to be 1–2 %. The male-to-female ratio is 3:2, and the age of presentation is closer to a mean of 60 years old if trauma etiologies are excluded [6].
Most HAAs are atherosclerotic, accounting for approximately 30 % of cases. Arterial dysplasia, trauma, PAN, and biliary diseases have also been implicated in the formation of HAA [6]. Rare cause is post vascular endothelial growth factor (VEGF) receptor tyrosine-kinase inhibitor (RTKI) therapy in a female patient with I131-refractory, differentiated, metastatic thyroid cancer [14]. In a Mayo Clinic series of 36 true HAAs, the most commonly associated comorbidity was hypertension, which occurred in 72 % of patients [15].
Superior Mesenteric Artery Aneurysms (SMAAs)
SMAAs appear to affect men and women equally, with most patients presenting at ages older than 50 years. Incidence based on autopsy studies is reportedly 1 in 12,000 to 1 in 19,000 [6].
The most common etiology of SMAAs continues to be infection; mycotic etiologies account for 60 % of all SMAAs. Other conditions related to true SMAA formation include atherosclerosis, connective tissue disease, pancreatitis, and trauma in the development of SMA pseudoaneurysms. [6]
Celiac Artery Aneurysms (CAAs)
Prevalence of CAAs based on autopsy review is about 1 in 8000. The majority of CAAs are found in men (66 %) and are discovered in patients at an average age of 56 years. Historically, the primary cause of CAAs was infection, while in contemporary clinical experience, atherosclerosis and congenital or developmental medial defects are main etiologies of CAAs [6].
Gastric Artery Aneurysms (GAAs) and Gastroepiploic Artery Aneurysms (GEAAs)
The etiology of GAAs and GEAAs has been attributed to atherosclerosis in 30 % of cases, trauma in 25 %, and infection in 15 %. Comorbid conditions include peptic ulcer disease, vasculitis, and pancreatitis. There is a reported male predominance, with initial presentation in the sixth or seventh decade of life. The majority of these aneurysms are located along the left or right gastric artery.
Pancreaticoduodenal Artery Aneurysms (PDAAs) and Gastroduodenal Artery Aneurysms (GDAAs)
Both are male predominant diseases with a ratio of 4:1 over female and present in the sixth decade of life. Factors associated with the formation of PDAAs include atherosclerosis, local infection, trauma, pancreatitis, FMD, congenital anomalies, and compression of the celiac axis by the median arcuate ligament (MAL) [8, 16]. Of 131 PDAAs reported between 1895 and 2014, 62 % were associated with occlusion or stenosis of the celiac axis, with 16 % attributed to compression by a MAL [17].
Atherosclerosis and pancreatitis are the two most common risk factors for GDAAs. The pancreaticoduodenal artery is the main collateral pathway between the celiac axis and the SAM. Increased blood flow in the pancreaticoduodenal artery, as compensation for occlusion or stenosis of the SMA or celiac axis, may cause a GDAA. [3, 18] In a review of the English literature over a 25-year period from 1970 to 1995, pancreatitis was found to be the most common associated condition with GDAAs accounting for 47 % of all cases followed by ethanol abuse (25 %), peptic ulcer disease (17 %), and cholecystectomy (3 %) [3]. As for pseudoaneurysms, inflammation with the most common cause being pancreatitis [19].
Inferior Mesenteric, Jejunal, Ileal, and Colic Artery Aneurysms
Connective tissue disorders that may contribute to the etiology of these aneurysms include Marfan’s syndrome, EDS, FMD, hereditary hemorrhagic telangiectasia, Osler-Weber-Rendu disease, and Kawasaki’s disease. Other described etiologies may include alpha one antitrypsin deficiency, infections (specifically tuberculosis), trauma, or iatrogenic injury during previous procedures [11]. A large series from the Mayo Clinic reported eight patients with these mesenteric branch aneurysms, noting that most of the patients had a significant smoking history and used alcohol [20].
Among 54 IMAAs reported between 1861 and 2012, atherosclerosis is the most common cause, accounting for 41 %. Other reported causes are mycotic, polyarteritis nodosa, Takayasu’s disease, iatrogenic, aortitis, segmental mediolytic arteritis, tuberculous, Behcet’s disease, and neurofibromatosis [10].
The common causes of middle colic artery aneurysm are atherosclerosis, angiodysplasia, arteritis, and infection. Among these, segmental arterial mediolysis (SAM) is the most common. [9] The etiologies of jejunal and ileal artery aneurysms remain poorly defined.
Rupture Risk
Rupture rate of VAAs largely depends on aneurysm location, shape, size, and etiology. Aneurysm rupture in the setting of circulatory collapse is a devastating clinical scenario, with perioperative mortality rates reported from 20 to 70 % [6]. The risk of rupture seems higher for pseudoaneurysms than for true aneurysms, and ruptures are more frequent in the hepatic artery (80 %), pancreatic arteries (75 %), and SMA (38 %).
Splenic Artery Aneurysms (SAAs)
SAAs have a relatively low rupture rate of less than 2 % in cases not associated with pregnancy. Although rare, the mortality for ruptures in nonpregnant patients is approximately 25–36 % [1, 3]. This rate nearly doubles in pregnant patients (65–75 %), with fetal mortality of 95 %, [21] and patients with portal hypertension (>50 %) [22]. Colonoscopy was the procedure reported most frequently associated with rupture. Common medications associated with rupture included anticoagulants, thrombolytics, and recombinant granulocyte-colony stimulating factor. Portal hypertension is an additional significant risk factor, associated with nearly 20 % of all SAA ruptures [6].
Other Splanchnic Aneurysms
Among all VAAs, HAAs have the highest rate of rupture, with reported rate varied from 60 to 80 %; mortality rates associated with rupture are high, ranging from 20 to 70 %. Rupture rates are 38–50 % for SMAAs with an associated mortality rate of 30–90 %. Rupture rates range from 10 to 20 % for CAAs, with a mortality of 50 %. It is 68 % for PDAAs, 56 % for GDAAs, and 90 % for GEAAs. The gastrointestinal or biliary tract is the site of rupture in 65 % of cases, whereas rupture into the retroperitoneal space occurs in 35 % of cases. In one series, 75 % of PDAAs presented as ruptured, with 50 % mortality. The mortality rate with rupture of a GDAA is about 40 %, with the highest mortality rate comes from rupture into the duodenum approaching 21 %. Less frequently, patients with GDAA can present with retroperitoneal or intraperitoneal bleeds with a 19 % mortality rate [6, 18].
Diagnostic Considerations
A thorough history including predisposing factors and past surgical intervention and physical examinations is essential for diagnosis. A pulsatile abdominal mass with or without a bruit on auscultation can be the sole warning sign and should initiate prompt diagnostic workup to preclude the worst outcome. Noninvasive imaging studies such as duplex ultrasound (DUS), CTA or MRA, or contrast arteriography are indicated in symptomatic patients suspicious for VAA. In asymptomatic patients the diagnosis of VAA is established only when imaging studies are performed to investigate other abdominal pathologies. It should be kept in mind that investigations that involve exposure of the fetus to radiations should be avoided or kept to a minimum.
Duplex Ultrasound (DUS)
DUS is a useful tool in the initial assessment of a suspected VAA, especially in pregnant patients to evaluate size of the aneurysm, mural thrombosis, as well as arteries proximal and distal to the aneurysm. It is very useful during long-term follow-up of small aneurysms treated conservatively. In the diagnostic evaluation of unstable patients, the use of a CT scan, MRI, or angiography has a limited role primarily because of their time-consuming nature, and the use of bedside ultrasound to detect intra-abdominal free fluid aids in the diagnosis of the less common causes of antepartum hemorrhage caused by rupture of a VAA [23]. However, this technique is operator dependent, and the detection of VAA might be missed in obese patients and those patients with visceral branch aneurysms or with considerable bowel gas.
Computed Tomography Angiography (CTA)
CTA has the advantage of being noninvasive and localizing the aneurysm with its relations to surrounding structures, size measurements, and evaluation of collateral flow and identifying areas of bleeding masking an aneurysm and is necessary for procedure planning (Fig. 34.5). Usually, the diagnosis of a VAA can be made with cross-sectional imaging, especially in visceral branch aneurysms. In patients with pancreatitis, CT scan can reveal a homogeneously enhancing structure within or adjacent to a pseudocyst which is highly suggestive of an associated pseudoaneurysm.
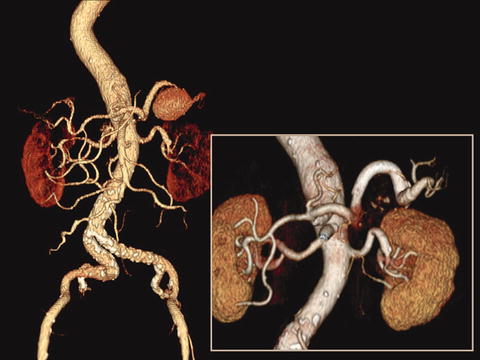

Fig. 34.5
Computed tomography angiography prior and after treatment of splenic artery aneurysm by stent graft
Magnetic Resonance Angiography (MRA)
MRA is an alternative examination, but it often does not provide the same detail as a CTA. In patients with renal failure, MRA is usually required for operative planning.
Selective Arteriography
Nowadays, catheter-based contrast angiography is seldom served as a diagnostic modality (Fig. 34.3). Instead, it is more frequently used in combination with percutaneous endovascular therapy of the VAA or diagnosis of visceral branch aneurysms, which are difficult to define the related anatomy with noninvasive imaging studies. Selective mesenteric angiography is a prerequisite to delineate the anatomy upon proceeding with endovascular treatment, which includes location of the aneurysm, inflow and outflow, collaterals, and tortuosity of the involved arteries. However, VAAs may not be visualized when mural thrombus or considerable thrombus present within the aneurysm.
Laboratory Studies
Laboratory results can be unremarkable in most of patients with VAA. Considering the variety of etiologies, standard white blood cell count, serum inflammatory markers, erythrocyte sedimentation rate and C-reactive protein, antibody and hepatitis panels, tuberculin skin testing, arteriography, and biopsy may be informative in diagnosing the etiology.
Treatment Modalities
The purpose of treatment of VAA is to exclude the aneurysmal sac from the systemic circulation while ideally preserving distal blood flow, while in case of rupture, the aim is immediate resuscitation and cessation of bleeding. Due to lack of natural history and prospective studies, there is no consensus regarding treatment guidelines for VAAs. Current treatment modalities include conservative treatment and open surgical or endovascular therapies. The choice of the therapeutic option is made on individual basis and depends on the symptom, the location of the aneurysm, and general condition of the patient and the risk of organ ischemia.
Observation
Observation is reserved for small aneurysms with relatively low risk of rupture. However, close imaging surveillance is required for early detection of rapid expansion in certain type of VAAs. In the report from the Mayo Clinic, of 168 patients with SAAs who underwent observation for a mean period of 75 months, only 10 % of the half patients monitored with serial imaging were noted to have aneurysm growth averaging 0.06/cm/year. No rupture or other complications related to the SAAs occurred, and only 3 of the original 168 patients (1.8 %) required intervention due to aneurysm growth [24]. The Cleveland Clinic reported similar results after reviewing 66 SAAs with observation; the average growth rate was 0.2/mm/year over 3.1 follow-up years. There were no ruptures or other complications attributed to the aneurysms in the observed group [25].
Indications for Interventions
Indications for interventions for VAAs are considered to be asymptomatic VAAs > 20 mm in size, rapidly increasing in size during surveillance ≥ 0.5 cm/year, symptomatic and ruptured VAAs, and pseudoaneurysms. Pancreaticoduodenal aneurysms are prone to rupture and should be treated at any size diameter. Ruptured VAAs should be treated emergently. In addition, because of the high risk of rupture, regardless of size, mycotic aneurysms, female patients of childbearing age or pregnant, or orthotopic liver transplant recipients are indicated for intervention for SAA; SMAA, CAA, multiple or non-atherosclerotic HAAs, and other branch aneurysms require intervention [5, 15]. Of all the mesenteric branch artery aneurysms, colic aneurysms are the most likely to rupture; therefore, they should be considered for intervention at an early stage [20].
Open approaches are reserved for cases with tortuous, angulated, or short vessels which are not amenable to the endovascular approach, those who require preservation of end-organ perfusion [11], mycotic aneurysms [26], or those who fail to endovascular intervention. Endovascular treatment is preferred for anatomically suitable patient with a hostile abdomen or abdominal sepsis, pancreatic inflammation, and high surgical risk.
Open Surgical Techniques
Treatment of VAAs with open surgery has been established as a safe and durable standard. Currently open repair is primarily researched for aneurysms involving branch vessels or bifurcation of critical vessels that cannot be incorporated with stent grafts or sacrificed with coil embolization or liquid agents. This is often the case of mid SMA trunk aneurysms or distal hepatic artery aneurysms. An open surgical approach allows for direct visualization of end organs and concomitant disease processes and is useful for determining the need for arterial reconstruction if intestinal viability is compromised. Open techniques include aneurysmectomy, aneurysmal ligation with or without bypass, and end-organ resection. The choice of technique depended on anatomic localization and the need for downstream revascularization which is largely dependent on the adequacy of collaterals. Some authors suggested to revascularize both the celiac artery and SMA whenever feasible. Intraoperative duplex ultrasound scanning during visceral revascularizations can help to optimize technical success and outcome [27]. Expeditious ligation of both the proximal and distal branches is generally performed without the need for reconstruction in the case of frank rupture and hemodynamic collapse and often during elective cases if adequate collateralization to end organs is present. Any small bowel ischemia may require resection. Minimally invasive surgical approach includes laparoscopic clipping as well as robotic assisted interventions [6].
Endovascular Techniques
Endovascular therapy has become the primary treatment modality whenever possible. The advent of minimally invasive endovascular therapy has provided an alternative to surgery in anatomically suitable patient in whom VAA needs to be treated, with the early benefits of reduced hospital stay and faster recovery. The most commonly used endovascular methods are coiling to induce thrombosis and stenting to exclude the aneurysm; access via the right femoral or left brachial artery is preferred. The use of endovascular approaches continues to expand with modern techniques and the development of endovascular materials. Endovascular management of ruptured VAAs has also been reported as a feasible option but should only be used in selective stable cases. However, some anatomical conditions may constrain these techniques, such as vessel tortuosity, aneurysm of the artery supplying an organ that has multiple arterial sources, main branch originating from the aneurysm, etc. Potential complications including visceral ischemia caused by sacrifice of the involved visceral artery, coil migration with resultant embolization of non-treated artery, end-organ thrombosis, vessel recanalization, persistence of the aneurysm, stent thrombosis, or restenosis should be taken into consideration. Prolonged imaging follow-up is also required for these patients since long-term durability after endovascular treatment is not well understood.
Transcatheter Embolization
Reported embolic materials include nitinol coils (Fig. 34.6), cyanoacrylate glue, thrombin, and even ethyl alcohol. The rate of glue polymerization can be altered for precision targeting using ethiodized oil dilution. A 5 F, 6 F, or 7 F long sheath is crucial to accommodate catheter to a target vessel; a 3 F microcatheter is useful for interventions in the distal aspect of an arterial bed for selective deployment of embolization material. Both true and pseudoaneurysms of the splanchnic circulation can be managed with embolization. Endovascular exclusion to ablate both inflow and outflow can be achieved by placement of coils first distally to the sac and then proximally to the sac. For aneurysms supplied by terminal branches, the sac can be directly injected or coiled [6]. Coil embolization is a well-known technique for the treatment of saccular aneurysms, but it can become challenging in wide neck; therefore, self-expandable stent or covered stent-assisted coil embolization techniques were used in the treatment of such aneurysms such as SAA [28] and CAA [29] to allow selective embolization of the aneurysm sac and blood supply to the distal artery and avoid migration of the coils which may lead to distal embolization. Also, proximal embolization alone should not be performed because the aneurysm may recruit a robust vascular supply in a retrograde manner. Clipping, coiling, and thrombin injection may also be achieved laparoscopically or robotically in appropriately selected patients [11].