Symptom
Number of patients (%)
Abdominal pain
231 (78)
Nausea
27 (9.1)
Vomiting
21 (7.1)
Ileus
11 (3.7)
Bloody stool
10 (3.4)
Diarrhea
8 (2.7)
Emaciation
6 (2.0)
Asymptomatic
47 (16)
On physical examination, patients are usually tender to palpation in the epigastrium or left upper quadrant. Although Froment et al. [45] reported an audible bruit in 17 % of their patients, it was present in only 3.0 % in the review by Luan et al. [11] In a series of 27 patients, recently reported by Kim and associates, periumbilical tenderness was found in 33 % of patients and none had rebound tenderness or peritonitis [41].
Celiac Artery Dissection (CAD)
Approximately one half of the patients with CAD are asymptomatic due to good collateral flow from the SMA [46]. The symptoms are usually nonspecific epigastric pain or postprandial abdominal pain. Sudden onset of epigastric or subcostal pain that resolves spontaneously within 1–5 days is an important sign; it is usually caused by irritation of the celiac nerve plexus or initial contained rupture of the aneurysm [47]. In some patients, an abdominal systolic bruit is present [48], although it is a rare finding that may be caused by associated SMA stenosis [47]. The celiac trunk divides into the left gastric artery, the splenic, and the common hepatic arteries (see Chap. 2 on splanchnic artery anatomy), thus all organs downstream of the dissected celiac trunk may suffer from ischemia depending on the involvement and compromised portion of the branch arteries. The clinical picture may include ischemic pancreatitis, gangrenous cholecystitis, ischemic gastropathy, liver ischemia, splenic ischemia with infarctions [49], or a life-threatening hemorrhage due to a rupture of the dissected artery or aneurysm [25, 27, 48, 50–54].
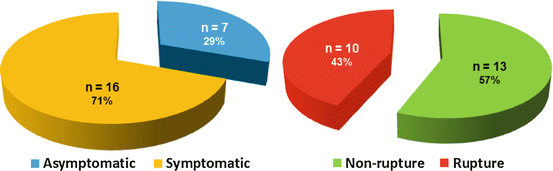
Of the reported 23 cases with hepatic artery dissection (HAD), 71 % have been symptomatic, typically presenting with pain in the epigastrium and right subcostal space (Fig. 31.1). Rupture with abdominal pain and shock occurred in 43 % of cases and rapidly caused death in all cases [29, 30]. Symptoms of splenic artery dissection are usually atypical and may also cause rupture and death of the patient.
The physical examinations and laboratory findings of most patients have been unremarkable except for epigastric tenderness [27, 51, 52]. However, spontaneous CADs must not be overlooked since complications are severe and include extension of the dissection into adjacent vessels, expansion of the false lumen, hemorrhage or organ ischemia, and the development of a celiac or hepatic artery aneurysm. Expansion of a false lumen may lead to rupture or it can result in poor distal perfusion due to compression of the true lumen [8]. Signs of acute liver ischemia are poor prognostic factors and they are associated with death in more than 40 % of the reported cases [47].
Renal Artery Dissections (RAD)
RAD can present either acutely or chronically; the clinical manifestations may vary from sudden onset of severe, persistent, and poorly controlled hypertension to acute flank pain ipsilateral to the site of dissection. Renal ischemia or infarction is a common finding in the acute setting; it occurs in approximately 40 % of the patients [55]. Nonspecific presentations include groin and/or testicular pain, headache, nausea, vomiting, fever, dysuria, hematuria, and blurry vision and these often lead to a delay in diagnosis [55–61]. Chronic RAD is typically encountered during evaluation for renovascular hypertension [56]; either it is asymptomatic or it is a cause of renovascular hypertension [62, 63]. Beroniade and colleagues described three different clinical sequelae of RAD: silent with no apparent ramifications, acute occlusion leading to renal infarction, and chronic dissection leading to renovascular hypertension [16].
Etiology
Compared to aortic dissection which is more common and widely studied, the underlying causes of IVAD are less well known. Possible etiologic factors of IVAD include arteriosclerosis, fibromuscular dysplasia (FMD), inflammatory or infectious disease, cystic medial degeneration (Marfan syndrome), segmental mediolysis arteriopathy, elastic tissue vasculitis, connective tissue disorders (Ehlers-Danlos syndrome) or vasculitis (giant cell arteritis, Takayasu arteritis, and polyarteritis), trauma including iatrogenic injury, hypertension, pregnancy, and a previous surgical history [10, 13, 19, 43, 48, 54, 64–74]. In cases with neither serologic evidence of vasculitis, connective tissue disorders, and inflammatory disorders nor traumatic injury including iatrogenic injury, dissections are classified as “spontaneous.”
Superior Mesenteric Artery Dissection (SMAD)
Compared to other IVADs, the etiology of SMAD has been studied the most extensively. SMAD is reported to be most prevalent in Asian (n = 222), followed by North American (n = 36), European (n = 29), and South American (n = 1) countries; [11] also, the majority of recent English language publications are from China [24, 39, 40, 74, 75] and South Korea [5, 41, 44]. Given the paucity of reports from the Western countries, this may reflect a possible genetic predisposition to SMAD in the Asian population. Rare causes of SMAD included strangulation due to seat belt injury [65, 76], iatrogenic injury such as contrast passage-induced or aggravated dissection during angiography or stent placement [42, 77–79], dissection after pancreaticoduodenectomy [80], or liver transplantation [81].
With respect to spontaneous isolated SMAD, Solis et al. found that shearing stress was an important mechanism because dissection commonly begins 15–30 mm from the orifice of the SMA, right between the fixed retropancreatic portion of the artery and the more distal mobile portion [65]. On a computer-simulation model, Park et al. [43] observed abnormal mechanical stresses on the anterior wall of convex curvature of the SMA and suggested this might be another possible etiology for dissection. Gender, social habit, and comorbidity may also relate to SMAD [43, 74]. In a series of 51 patients with spontaneous isolated SMAD compared with 38 patients with combined aortic and SMA dissection, Park and colleagues found that spontaneous isolated SMAD was more common in men (90 % vs 71 %, P = .02) and occurs less frequently with hypertension (31 % vs 66 %, P = .001) [43]. Similar observations were also reported by Li et al. [74]. Spontaneous isolated SMAD was reported to be more frequent in patients with intra-abdominal cancers [43] and in those with a smoking history [74].
Celiac Artery Dissection (CAD) and Hepatic Artery Dissection (HAD)
Spontaneous CAD has typically been associated with hypertension, arteriosclerosis, degeneration of the arterial wall, trauma, pregnancy, and arteriopathy; however, most cases can present without identifiable risk factor [7, 46, 47, 69, 82–84]. Some patients have type IV Ehlers-Danlos syndrome (EDS) [21, 85], also known as the vascular type; it is a rare connective tissue disorder with autosomal dominant transmission (McKusick catalog number 130050) caused by mutations in the COL3A1 gene, resulting in structural alteration of type III collagen [86]. Rare causes of CAD include microtrauma caused by a sudden increase in abdominal pressure, such as weight lifting [47, 84, 87], and rupture of a pancreaticoduodenal arcade aneurysm [88]. Isolated HAD was observed to develop during pregnancy [89] or during selective arteriography done for radioembolization [90].
Renal Artery Dissection (RAD)
RAD is predominantly associated with FMD, especially in chronic cases. Other causes include EDS and atherosclerosis; [2, 55–58, 60] however, many cases present in otherwise healthy individuals [91–94]. Although FMD itself has a female predominance [2], the largest published series of 24 RADs from Mayo Clinic showed a 10:1 male-to-female ratio; [2] this and other publication suggest RAD is a male-predominant disorder [58, 60]. In the first systematic study that included 17 patients, RAD patients were more likely to have history of hypertension, cancer, and connective tissue disorders (P < .001), and less likely to have obesity (BMI ≥30 kg/m2), compared with the general population [60]. RAD occurred most frequently in middle-aged men, with a history of hypertension, flank pain, elevated creatinine, and uncontrolled blood pressure at presentation. Associated connective tissue disorders in this study included FMD, EDS, polyarteritis nodosa, gout, or arthritis.
Different types of trauma have also been reported to cause RAD, ranging from blunt trauma to playing football and running marathons [91, 93–96] to iatrogenic trauma due to mal-deployment of endograft during endovascular aneurysm repair (EVAR) [97]. Rare causes included subadventitial angioma of the renal artery [98, 99], cocaine abuse [100], and extracorporeal shock wave lithotripsy [101].
Diagnostic Considerations
Since the clinical presentation is diverse and the disease is rare, early diagnosis of IVAD is rarely established. A thorough history taking and physical examination are essential. Noninvasive imaging studies such as duplex ultrasound (DUS), CTA, MRA, or contrast arteriography are indicated in symptomatic patients suspicious for IVAD. In asymptomatic patients, the diagnosis of IVAD is established only when imaging studies are performed to investigate other abdominal pathologies. Laboratory studies are usually within normal ranges unless ischemic changes of splanchnic organs develop.
Duplex Ultrasound Scanning (DUS)
DUS is a useful tool in the initial assessment of a suspected IVAD or in those patients in whom renal function is compromised. Duplex scanning of the visceral arteries may be helpful in identifying the intimal flap (Figs. 31.2 and 31.3) and the entry or reentry points [11]. DUS is used to assess the flow velocities and resistive index in the renal arteries as well as to evaluate end-organ vascularity. The intestinal wall can also be assessed with a high degree of accuracy by high-resolution transabdominal ultrasound. Transmural hemorrhage, inflammation, and necrotic thickening in the bowel wall can be imaged sonographically [102]. However, this technique is operator-dependent and does not provide the necessary detail to make treatment decisions, considering the anatomy of the affected visceral arteries and their branches, obese patients, patients with considerable bowel gas, and the diagnosis of IVAD may be missed as well. In patients with RAD, preoperative DUS can be performed to evaluate the size of the kidneys, measure the intraparenchymal resistive indexes, and detect renal infarcts which typically appear as wedge-shaped, hypoechoic lesions with absent blood flow on DUS.
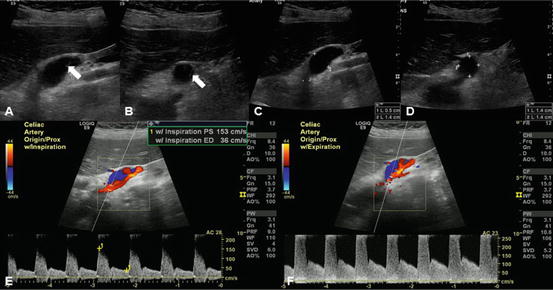
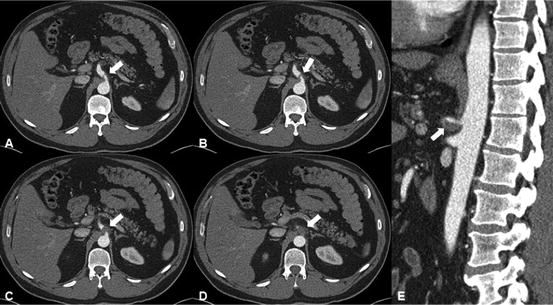

Fig. 31.2
Celiac artery dissection with high-grade stenosis in the proximal celiac artery and distal celiac artery aneurysm. Note changes in flow velocity with inspiration and expiration suggesting median arcuate ligament compression. (a, b) Arrow indicates intimal flap of the celiac artery dissection. (c, d) 1.4 cm celiac artery aneurysm that measured 2.7 cm in length. (e) Peak velocity measures 153 cm/s at inspiration. (f) Peak velocity measures 401 cm/s at expiration

Fig. 31.3
Celiac artery dissection (CAD) extending into the entire splenic artery. (a, b) Arrow indicates intimal flap of the CAD. Within the proximal celiac artery, there is an asymmetric filling defect suggestive of thrombosis. (c, d) Thrombosed false lumen. (e) Thrombosis involving the inferior aspect of the proximal celiac artery with moderate luminal narrowing on sagittal view
Computed Tomographic Angiography (CTA)
When an IVAD is suspected, CTA is the first-line diagnostic modality. CT imaging progressed by leaps and bounds in the past decade, especially with the introduction of interactive multiplanar reconstruction (MPR) /maximum intensity projection (MIP)/3-dimensional (3D) rendering [17, 18, 21, 68]. Plain computed tomography (CT) shows areas of high intensity if there is an acute thrombus in the false or true lumens. Dynamic enhanced CT is helpful to separate the true lumen from the false lumen by an intimal flap [36]. Modern multidetector CT enables imaging with excellent spatial and temporal resolution. In addition to being quick and accurate, it provides the detailed information including intimal flap, anatomy, mural thrombosis, intramural hematoma, true and false lumen, and compromised visceral arteries with accuracy [21, 39]. An intimal flap separating the proximal portion of the visceral arteries into true and false lumen is a definitive diagnostic finding (Figs. 31.2, 31.3, 31.4, and 31.5); infiltration of the fat surrounding the affected vessel is a well-known secondary sign of acute spontaneous dissection of a visceral artery.
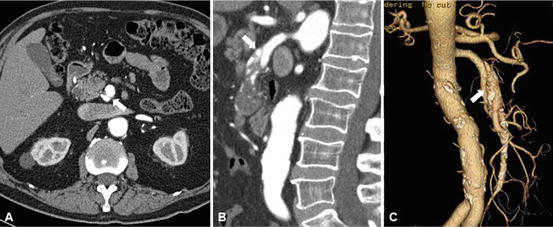
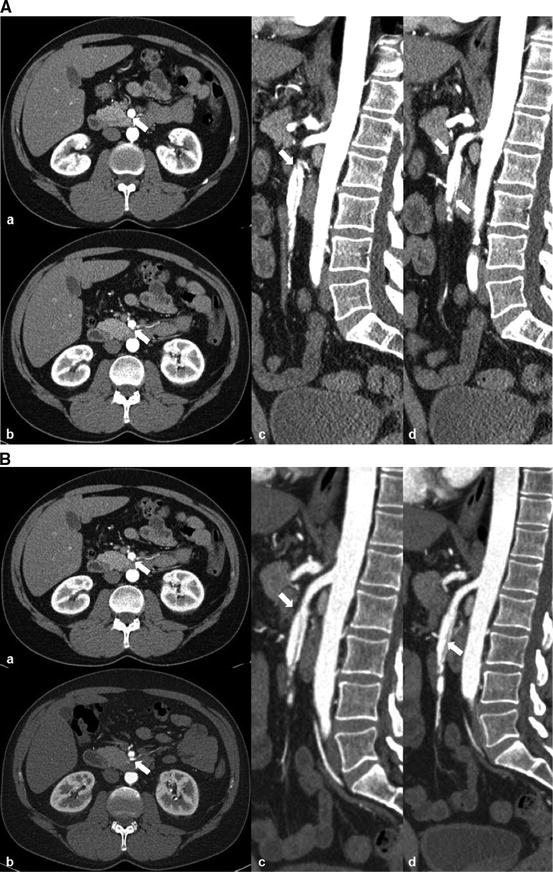

Fig. 31.4
Superior mesenteric artery dissection (SMAD) and aneurysmal dilatation, involving the mid SMA. Arrow indicates intimal flap of the SMAD. (a) Intimal flap of SMAD on axial view. (b) Intimal flap of SMAD on sagittal view; the aneurysm contains significant amount of mural thrombus proximally and completely thrombosed distally. (c) Intimal flap of SMAD on 3D reconstruction and moderate amount of peripheral calcification of SMA

Fig. 31.5
Superior mesenteric artery dissection. Arrow indicates intimal flap of the SMAD. (a) Dissection begins 4 cm distal to the origin of SMA and extends 5 cm into a jejunal branch. Mild aneurysmal dilatation at the level of the dissection measures 11 mm. (b) Follow-up CTA at 4 years, stable SMAD, dilatation with the SMA measuring about 13 mm. a, b axial view, c, d sagittal view
In patients with SMAD, the dissection usually starts within 5–50 mm from the original site [3, 9, 24, 26, 39, 65], where the SMA transitions from an immobile status to a mobile status along the inferior pancreatic margin. At this site, as mentioned before, there is increased shearing force, which may contribute to the intimal tear. The length of SMAD usually varies from 10 to 160 mm [3, 35, 39].
Reviewing CT findings of 34 patients, Jung et al. pointed out that intimal flap, thrombosed false lumen, and aneurysmal dilatation are the most common findings of IVAD [9]. In the symptomatic group, the mean distance from the orifice of the SMA to the intimal flap in 17 SMADs was 19.7 mm (range 6–43 mm); the mean values of the largest diameter and the length of the dissecting artery were 14.7 mm (range 8.4–50 mm) and 85.4 mm (range 33–180 mm), respectively. In four patients with CAD, the mean distance from the orifice to the site of dissection was 10.8 mm (range 9–12 mm). The mean values of the largest diameter and the length of the dissecting artery were 14 mm (range 11–20 mm) and 27.8 mm (range 21–40 mm), respectively. The largest diameter and the length of the dissecting artery in the asymptomatic group were significantly smaller than those of the symptomatic group.
In a recent study of 24 SMADs, an intimal flap which separated the SMA into a true lumen and a false lumen was observed in 83 % of patients; increased SMA diameter occurred in 63 %, 75 % had stenosis of the true lumen, thrombosed false lumen was seen in 63 %, and dissecting aneurysm was present in 30 % [75]. In another study of 27 SMADs, a patent false lumen with both entry and reentry was documented in 19 % of the patients, and partially or completely thrombosed false lumen presented in 81 %; the mean percent of compression of the true lumen occurred in 62 % [41].
Verde et al. reported 24 CADs and 18 SMADs in 38 patients; 30 % of the dissections diagnosed with interactive MPR/MIP/3D rendering were missed on standard imaging planes, and the authors suggested that interactive MPR/MIP/3D rendering can increase diagnostic sensitivity particularly in the setting of pain without any other findings to explain the symptoms [21]. Currently, there is a multitude of classification systems that have been used to distinguish the different types of dissections in the literature and no system is accepted as the gold standard. Based on CT findings, Suzuki et al. [18] classified SMAD into four types: (1) an intimal flap, (2) hematoma of the false lumen or intramural hematoma, (3) an enlarged SMA diameter, and (4) increased attenuation of the fat surrounding the SMA. The authors suggested that the increased attenuation of the fat was an early diagnostic finding.
Sakamoto et al. [19] classified SMAD into four types based on the patency of the false lumen: type I, patent false lumen with both entry and reentry; type II, “cul-de-sac”-shaped false lumen without reentry; type III, thrombosed false lumen with an ulcer-like projection (ULP); and type IV, completely thrombosed false lumen without a ULP. Zerbib et al. [26] described a modified Sakamoto’s classification adding the type of “dissecting aneurysm” and the type of “thrombosed SMA.” However, the Sakamoto’s classification does not take into consideration the patency of the true lumen, which is important to evaluate the mesenteric blood supply and make therapeutic decision.
Cho et al. [3] divided SMAD into two types: (1) double lumen with an intimal flap, with a patent or closed false lumen, and (2) intramural hematoma, which was further subcategorized depending on the presence/absence of SMA stenosis [3]. Dong et al. [24] reported three common findings for SMAD: (1) no identified reentry, (2) patent or partially/completely thrombosed false lumen with larger diameter compared with that of the true lumen, and (3) true lumen compressed by the false lumen.
Most recently, Li et al. [74] proposed a modified morphological classification with subtypes depending on the patency of the true lumen. The following five types were identified: type I, patent false lumen with both entry and reentry; type II, “cul-de-sac”-shaped false lumen without reentry (IIa, patent true lumen; IIb, severe stenosis of the true lumen; IIc, occlusion of the true lumen); type III, thrombosed false lumen with a ULP (IIIa, patent true lumen; IIIb, severe stenosis of the true lumen; IIIc, occlusion of the true lumen); type IV, completely thrombosed false lumen without a ULP (IVa, patent true lumen; IVb, severe stenosis of the true lumen; IVc, occlusion of the true lumen); and type V, dissecting aneurysm. The subtypes of a, b, and c were determined by true lumen residual diameter (TLRD); a dissecting aneurysm of the SMA was defined as an increase in diameter of more than 50 % compared with the normal part of the SMA. According to this classification, of 42 patients studied, 19 % were type I SMAD, 10 % type II, 26 % type III, 38 % type IV, and 7 % type V.
In another classification system, Luan et al. [22] grouped SMAD into four types based on the location of dissection: type A, the dissection was localized at the curved part of the SMA and extended proximally to the SMA ostium; type B, the dissection was limited to the curved part of the SMA; type C, the dissection was localized at the curved part and extended distally, but the ileocolic artery or distal ileal artery was not involved; and type D, the dissection was localized at the curved part and extended distally to the ileocolic artery or distal ileal artery. In their study of 20 patients, the distributions of SMAD in the four types were 25, 30, 10, and 35 %, respectively. Due to the slender nature of the SMA, the imaging examination cannot always detect the entry or reentry site in SMA, and some of the dissections show indefinite distal dissection into the multiple branches. Thus, dissected lesions cannot always be categorized using these classification systems, and the morphological features of the dissection cannot completely predict the outcome and prognosis of the dissection as well.
Magnetic Resonance Angiography (MRA)
MRA is an alternative examination, but it often does not provide the same detail as a CTA. The recognition of the gadolinium-induced nephrogenic system fibrosis substantially limits today the use of contrast-enhanced magnetic resonance imaging (MRI) in patients with a glomerular filtration rate of <60 ml/min. Case report showed that MRI without intravenous contrast media was possible to identify renal infarction by using diffusion-weighted image (DWI) sequence and RAD by using a 3D inflow inversion recovery sequence. This is particularly useful in patients with renal failure [23].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree