Fig. 40.1
Pathologic specimen of a bicuspid stenotic calcified aortic valve
40.1.2 Clinical Presentation
The clinical presentation of aortic valve stenosis depends upon the severity of the obstruction. The measure of severity of the obstruction is based upon the pressure drop across the valve during systole. In trivial aortic stenosis, the pressure difference is 0–25 mmHg; in mild stenosis it is 26–50 mmHg. In moderate stenosis, the pressure difference is 51–79 mmHg, while it is >80 mmHg in severe stenosis.
Most patients with aortic stenosis are asymptomatic and are diagnosed because of the presence of a cardiac murmur. Rarely, syncope is the presenting sign of aortic stenosis. Some infants with severe aortic stenosis can present with low cardiac output, severely reduced ejection fraction, shock, and/or congestive heart failure.
Aortic insufficiency usually presents with a murmur. Severe or progressive aortic insufficiency may present with fatigue, shortness of breath, exercise intolerance, orthopnea, and/or paroxysmal nocturnal dyspnea.
40.1.3 Physical Examination
The apical impulse may be increased for patients with moderate or severe stenosis. There may be a thrill palpable at the base of the heart and over the carotid arteries. The first heart sound is normal and the second heart sound is normal. With aortic valve stenosis (but not with supravalvar or subvalvar stenosis) an ejection click will be heard immediately after the first heart sound.
The murmur of aortic stenosis is systolic and crescendo decrescendo or “diamond shaped.” It is best heard along the left sternal border and radiates to the right upper sternal border. Some patients with aortic stenosis have associated aortic insufficiency, and, if that is the case, a decrescendo diastolic murmur will be audible.
Aortic insufficiency is characterized by a decrescendo murmur that begins immediately after S2.
40.1.4 Echocardiography and Cardiac Catheterization
Using echo/Doppler techniques, it is possible, noninvasively, to estimate the transaortic pressure gradient. Using echocardiography, one can assess left ventricular wall thickness, which also is a reflection of the severity of the stenosis.
Echocardiography and Doppler can confirm the presence of aortic insufficiency and is useful in determining the severity of the leakage. Using these techniques, one can determine the regurgitant fraction, the left ventricular systolic and diastolic dimensions, and the left ventricular ejection fraction.
40.1.5 Treatment
Treatment of aortic valve stenosis involves manipulating the valve to reduce the stenosis. This can be accomplished by transvenous balloon dilation of the valve, open surgical valvotomy, or surgical valve replacement. Valve replacement includes several possibilities. The stenotic valve could be replaced with a homograft valve, a porcine valve, or a mechanical valve.
An additional option is the Ross procedure. In this operation the patient’s pulmonary valve is removed and sewn into the aorta in place of the stenotic aortic valve. A homograft valve is then used in the pulmonary position.
There are several approaches to the patient with significant aortic insufficiency. The options for valve replacement are the same as those discussed above for aortic stenosis. In addition, the surgeon can, in select cases, repair the valve.
Since patients with bicuspid aortic valve or aortic stenosis can develop an ascending aneurysm, repair of the aneurysm will be necessary if it is large.
40.1.6 Outcome
Aortic stenosis is a lifelong issue despite treatment. After balloon dilation or surgical valvotomy, there is a 40 % chance over the next 25 years that additional surgery will be necessary, usually to treat aortic regurgitation. If aortic valve replacement is done using a mechanical prosthesis, the patient will require lifelong anticoagulation therapy. If a tissue valve is used, it eventually will have to be replaced. In general the half-life for a tissue valve is 10–15 years.
Primary aortic valve insufficiency can be treated, in some cases, by repair of the leaking aortic valve. However, in most cases valve replacement will be necessary. The periprocedural mortality for these procedures is less than 5 %.
40.2 Coarctation of the Aorta
40.2.1 Pathologic Physiology
Coarctation of the aorta consists of a ledge-shaped protrusion of tissue into the aorta immediately opposite the insertion of the ductus or ligamentum arteriosum (Fig. 40.2). Frequently, there is an associated bicuspid aortic valve.
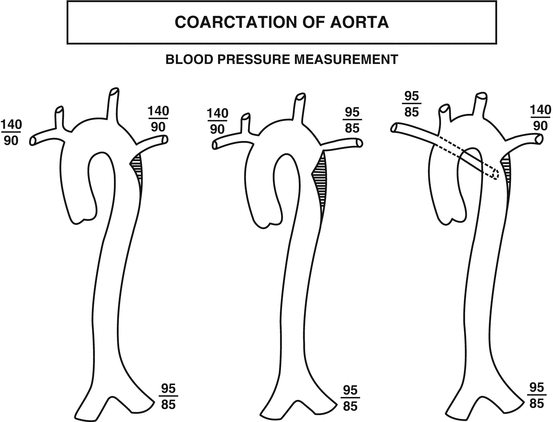





Fig. 40.2
Diagrammatic representation of anatomic variants of coarctation of the aorta. The left-sided figure is the most common form. The middle figure illustrates associated stenosis of the left subclavian artery and the right-sided figure demonstrates associated anomalous origin of the right subclavian artery. The blood pressure measurements illustrate how the anatomic variants will affect the blood pressure measurements (Reproduced or adapted from Driscoll, David, Fundamentals of Pediatric Cardiology, Lippincott Williams & Wilkins, 2006, with permission of the author and publisher)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


