Clinical Predictors of Improvement in Left Ventricular Performance with Cardiac Resynchronization Therapy
Luis A. Pires
Heart failure (HF) is characterized by progressive dilatation of the left ventricle (LV) and loss of contractile performance, together referred to as LV remodeling. It is not surprising, therefore, that the extent of LV remodeling1 and treatments aimed at reversing its course2,3,4,5 have a significant impact on the outcome of HF patients. Despite recent advances in the pharmacologic therapy of HF, morbidity and mortality remain high.6 This plus the increasing incidence of HF6 has recently led to a search for new treatment modalities, most notably the role of cardiac resynchronization therapy (CRT), precisely because it is thought to reverse the course of LV remodeling.7,8 Although the precise mechanism by which CRT improves patient outcome is not completely understood, but is beginning to be elucidated,9,10 a likely explanation is thought to be its significant impact on systolic and, perhaps to a lesser extent, diastolic LV performance observed both acutely and over several months.11 The results of several observational and randomized clinical trials have established CRT (via biventricular pacing), as a major breakthrough in the treatment of patients with moderate-to-severe HF, impaired LV systolic function, and intraventricular conduction delay.12 CRT improves quality of life and exercise capacity, reduces severity of mitral regurgitation (MR), reduces HF hospitalizations, and improves overall survival in patients who are already receiving optimal pharmacologic therapy.13 Despite the impressive results obtained from CRT in terms of both symptom improvement and reversal of LV remodeling, however, as many as 20% to 50% of patients do not respond to such therapy.14 None of the established CRT selection criteria (NYHA class, LV ejection fraction, and QRS duration) are able to adequately predict either acute or longterm response to CRT.15,16,17,18,19,20,21,22,23 On the other hand, improvement in LV performance, quantified primarily as reversal of LV remodeling, appears to be a strong predictor of patient outcome,24,25,26 similar to what is observed with drug therapy.2,3,4,5 Although far less is known about CRT and right ventricular (RV) performance, CRT also acutely improves RV performance,27,28,29 which, in turn, predicts adverse events.30 The focus of this chapter, however, will be on the effect of CRT on LV performance.
This chapter reviews predictors of acute and long-term improvement in LV performance in patients with chronic systolic HF and established indications for CRT. The latter part of the chapter will focus on patient groups for whom indications (and results) of CRT are less well established.
IMPROVEMENT IN LEFT VENTRICULAR SYSTOLIC PERFORMANCE WITH CRT
Improvement in LV systolic performance has been quantified, most commonly, in terms of changes in dP/dtmax, pulse pressure, stroke volume, LV ejection fraction (EF), LV endsystolic (LVESV) and end-diastolic (LVEDV) volumes. Though earlier reports focused on invasive tools (pressurevolume analysis), echocardiography has now become the preferred method to assess global and regional changes in LV performance before and after CRT.31 Determination of the impact of CRT on LV systolic (and diastolic) performance, both acutely and long-term, greatly enhances our understanding of the potential mechanism(s) of action of CRT and the relationship between such improvement (and its absence) and long-term patient outcome. Yu et al.26 recently reported that, unlike improvements in clinical parameters such as NYHA functional class and exercise capacity, a reduction in LVESV (by ≥10%) was the only independent predictor of all-cause and cardiovascular mortalities over 3 to 6 months of follow-up in CRT-treated HF patients.27 The 87 (62%) CRT responders (i.e., those with a ≥10% reduction in LVESV) had significantly lower all-cause mortality (6.9% versus 30.6%, p = 0.0003), cardiovascular mortality (2.3% versus 24.1%, p < 0.0001), and heart failure events (11.5% versus 33.3%, p = 0.0032) than nonresponders (i.e., those with a <10% reduction in LVESV). From this and other studies,24,25,26 it is becoming clear that reduction in LV volumes and increase in LVEF are not only surrogate markers of a favorable response to CRT but may also be used as objective measures to predict long-term clinical outcome, thereby facilitating selection of patients who are most likely to respond to and benefit from therapy.
Acute Improvement in LV Systolic Performance
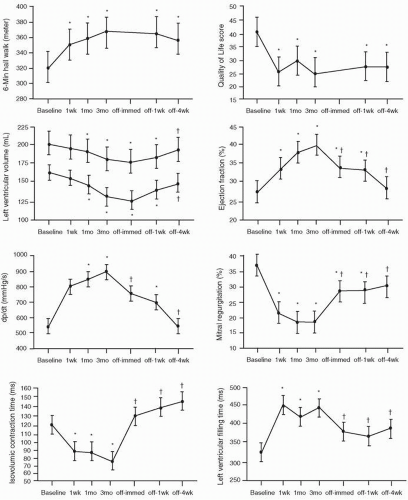
Several acute hemodynamic studies have shown that CRT results in immediate and sustained improvement in LV performance,32,33,34,35,36,37,38 which served as the background for subsequent clinical trials confirming the substantial benefits of CRT. CRT acutely enhances systolic function while modestly lowering energy cost,38 an important feature considering the adverse impact of pharmacologic therapies that enhance LV function at increased myocardial oxygen consumption.39,40 Improvement in stroke volume and cardiac output, expressed usually as changes in aortic pulse pressure, range from 7% to 15%.9, 11, 32,33,34,35,36,37,38 An acute increase of 13% to 21% in dP/dtmax, a reflection of global LV performance, has been reported.9, 11, 32,33,34,35 Similarly, acute reductions in LVESV and to a lesser degree LVEDV have been observed, often in parallel with changes in pulse pressure and dP/dtmax.9,11,13,38 Acute absolute (relative) increases of 4% to 10% (15% to 36%) in LVEF have been reported.11,13,28,29,37,41 Acute improvement in LV performance is maintained chronically,11,29,42 but such improvement disappears to a substantial degree with cessation of pacing.11,42 Yu et al.42 elegantly showed that several measures of LV performance progressively increased after both one week and three months of biventricular pacing. These measures of improvement gradually declined with cessation of pacing (Fig. 2.1). Moreover, such changes were paralleled by concordant changes in LV volumes, severity of MR, exercise capacity, and quality of life score. The fact that CRT-mediated gains in LV performance are lost with termination of pacing indicates that some of the derived benefits are purely pacing dependent (“electrical” phenomenon), and not the result of reversal of remodeling, which would imply actual structural changes.43 It should also be noted that the acute impact of CRT on LV performance noted in resting conditions, typical of the reported results,32,33,34,35,36,37,38 is not maintained under “stress” conditions,44 which raises the interesting possibility that, in some cases, this may account for the lack of response to CRT during longterm follow-up.14 The settings under which LV performance is determined should be taken into account in data analysis.
Predictors of Acute Improvement in LV Systolic Performance
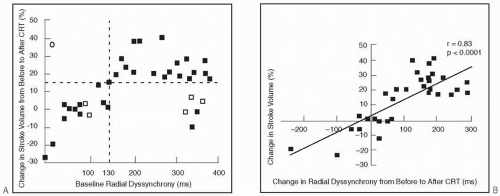
Predictors of acute improvement in LV performance with CRT have been reported by several investigators.28,45,46,47,48,49,50,51 Using various measures to define improvement in LV performance, responders (46% to 91%) were identified with sensitivities of up to 100% and similar specificities (Table 2.1). In general, traditional, readily available clinical variables, or patient demographics, have not proven useful. In one of the earlier reports involving 22 patients, Nelson et al.45 found baseline QRS duration and dP/dtmax to be weak predictors of increases in dP/dtmax (≥25%) and pulse pressure (≥10%) individually, but strong predictors when combined. The majority of studies, however, focused on various measures of interventricular and intraventricular dyssynchrony to identify predictors of acute improvements in LV performance (Table 2.1). Using radial strain imaging to quantify LV dyssynchrony and a cutoff value of ≥130 ms, Dohi et al.49 found a close relationship between improvement in stroke volume and baseline dyssynchrony (Fig. 2.2A) and change (reduction) in dyssynchrony after CRT (Fig. 2.2B). Suffoletto et al.51 reported similar results, including high sensitivity and specificity in predicting an immediate ≥15% increase in stroke volume with CRT. Moreover, these investigators found that in the 22 patients whose LV leads were positioned concordant with the site of latest mechanical activation by radial strain had a greater increase in LVEF from baseline (10±5%) than the 14 patients with discordant LV lead positions (6±5%, p < 0.05). These results emphasize the importance of proper LV lead position in determining CRT response.52,53 An excellent correlation between speckle-tracking and tissue Doppler determined dyssynchrony, defined as the time difference between septal and posterior wall peak strain,51 was observed. This is potentially an important finding given that the more commonly used tissue Doppler method may be more susceptible to passive translational motion or tethering,54 and thus subject to interpretive errors.31
The extent of myocardial viability in patients with ischemic cardiomyopathy appears to play an important role in both acute and long-term (see below) CRT response. Hummel et al.55 found a close relationship between acute (the day after CRT implantation) improvement in LVEF and stroke volume and extent of myocardial viability (perfusion score index) quantified by contrast echocardiography. These investigators showed that baseline intraventricular dyssynchrony and degree of resynchronization only weakly predicted acute LVEF improvement; resynchronization resulted in improvement in LVEF only in patients with significant tissue viability.55 These findings suggest that largely nonviable akinetic or dyskinetic LV segments contribute little to LV systolic performance, whether or not they are resynchronized. In an acute hemodynamic study involving 53 patients with moderate-tosevere HF, Van Gelder et al.56 found a significantly lower percent increase in dP/dt in patients whose LV lead tips were placed where transmural scar (MRI-detected hyperenhancement involving >50% of wall thickness) was present (14.5 ± 10.4) compared with sites remote from scar (27.5 ± 18.4) or where there was no scar (32.0 ± 20). They also found that the optimal V-V interval was significantly longer in patients with scar tissue, perhaps a reflection of scar-mediated slowing of conduction.
Other investigators have used combinations of arguably simpler baseline (LV contractility and mechanical dyssynchrony) and procedural (position and electrical delay of LV leads relative to RV leads) variables to construct a “response score” to predict hemodynamic improvement (and clinical outcome) of CRT-treated patients.57 These investigators found a highly significant association between response score (0 to 4 points) and the proportion of hemodynamic
responders to CRT (≥25% increase in dP/dt with pacing), with one point given to each of four analyzed variables: LV/RV lead distance > 10cm; LV lead electrical delay > 50% of QRS; dP/dtmax <600 mm Hg/s; and LV dyssynchrony >100 ms delay. They also observed a significant link between response score and overall patient survival. As pointed out by the authors, such response score was generated retrospectively; therefore its utility in predicting CRT response (acutely and chronically) must be validated prospectively.
responders to CRT (≥25% increase in dP/dt with pacing), with one point given to each of four analyzed variables: LV/RV lead distance > 10cm; LV lead electrical delay > 50% of QRS; dP/dtmax <600 mm Hg/s; and LV dyssynchrony >100 ms delay. They also observed a significant link between response score and overall patient survival. As pointed out by the authors, such response score was generated retrospectively; therefore its utility in predicting CRT response (acutely and chronically) must be validated prospectively.
The results of these studies (Table 2.1) are not without limitations, including relatively small number of patients, singlecenter enrollments, different definitions of measures of response, heavy focus on echocardiographic predictors, and, of course, lack of randomization of CRT. Moreover, other investigators did not identify any reliable predictor, among several potential parameters, of an acute improvement in stroke volume with CRT.44 None of these studies addressed whether or not these acute results can be used to predict or determine long-term clinical outcome. Limited data, on a small number of patients, suggest that acute improvement in LV performance (stroke volume and LVEF) leads to further short-term increase in LVEF29 as well as improvements in exercise duration58 and NYHA functional class.47,59 There exists no largescale data on the significance of acute improvement in LV performance and long-term outcome of CRT-treated patients.
Short- and Long-Term Improvement in LV Systolic Performance
Enthusiasm regarding the role of CRT as an adjunctive treatment modality in patients with chronic systolic HF grew when randomized clinical trials confirmed its substantial benefit on LV performance and reversal of LV remodeling.12 It is now widely believed that the overall positive impact of CRT on short- (3 months) and long-term (6 months or longer) patient outcome is attributed to its sustained, progressive enhancement in LV performance.7,8,11,25,29,42 through retiming (resynchronization) of dyssynchronous LV contraction.10 Aside from its immediate hemodynamic effect,11,32,33,34,35,36,37,38 CRT results in improvement in LV performance starting as early as one week,29, 42 and maintained over several years.60
Predictors of Improvement in Short-and Long-Term LV Performance
Several predictors of improvement in short- and long-term LV performance have been identified.29,61,62,63,64,65,66,67,68,69 Relying on various changes in LVEF or LV volumes to define response, the authors reported response rates of 40% to 85% with sensitivities of 72% to 100%, and specificities of 55% to 100% (Table 2.2). Non echocardiographic factors have not been particularly helpful as predictors. On a multivariate model of the combined data from MIRACLE and MIRACLE-ICD trials, in which 60% of the patients experienced a decrease in LVEDV, and 40% had an increase in LVEDV, Cappola et al.61 identified male gender, baseline BNP level, severity of MR, and baseline (smaller) LVEDV each as independent predictors of LV enlargement (remodeling).61 This suggests that female gender, lower BNP levels, less-severe MR, and larger LVEDV might be protective against LV enlargement. The observation that male gender attenuated the decline in LVEDV might explain, in part, the higher mortality rate in men with HF compared with
women.70 Mangiavacchi et al.62 identified nonischemic etiology of HF as the only independent predictor of a marked improvement in LV performance, defined as an increase of > 10 absolute percentage points in LVEF (observed in 39.7% of patients). This finding is in agreement with other studies in which a better improvement in LV performance was observed among patients with nonischemic LV systolic dysfunction who were treated with either beta-blockers71 or CRT.17,18,72,73 Similarly, mortality appears to be lower among HF patients with nonischemic cardiomyopathy treated either medically6 or with CRT.73 The better CRT-induced improvement in LV performance (and overall outcome) in patients with nonischemic etiology probably reflects the fact that CRT may not be able to resynchronize scar-laden myocardial segments, a finding in agreement with the reduced benefit of CRT in patients with substantial scarring or reduced tissue viability.41,55,74,75,76 Bleeker et al.74 found that patients with MRI-detected extensive posterolateral scarring, a favored site of LV lead placement, are unlikely to show improvement in LV performance regardless of the extent of baseline LV dyssynchrony. A higher overall scar burden, a larger number of severely scarred segments, greater scar density near the LV tip,75,76 or reduced tissue viability42,55 portend an unfavorable response to CRT in terms of changes in LV performance and in functional improvement (Fig. 2.3). Other investigators, however, found that, as long as acceptable LV pacing was achieved, placement of the LV lead at an akinetic (scarred) segment did not adversely impact acute hemodynamic or 12-month clinical response to CRT.77
women.70 Mangiavacchi et al.62 identified nonischemic etiology of HF as the only independent predictor of a marked improvement in LV performance, defined as an increase of > 10 absolute percentage points in LVEF (observed in 39.7% of patients). This finding is in agreement with other studies in which a better improvement in LV performance was observed among patients with nonischemic LV systolic dysfunction who were treated with either beta-blockers71 or CRT.17,18,72,73 Similarly, mortality appears to be lower among HF patients with nonischemic cardiomyopathy treated either medically6 or with CRT.73 The better CRT-induced improvement in LV performance (and overall outcome) in patients with nonischemic etiology probably reflects the fact that CRT may not be able to resynchronize scar-laden myocardial segments, a finding in agreement with the reduced benefit of CRT in patients with substantial scarring or reduced tissue viability.41,55,74,75,76 Bleeker et al.74 found that patients with MRI-detected extensive posterolateral scarring, a favored site of LV lead placement, are unlikely to show improvement in LV performance regardless of the extent of baseline LV dyssynchrony. A higher overall scar burden, a larger number of severely scarred segments, greater scar density near the LV tip,75,76 or reduced tissue viability42,55 portend an unfavorable response to CRT in terms of changes in LV performance and in functional improvement (Fig. 2.3). Other investigators, however, found that, as long as acceptable LV pacing was achieved, placement of the LV lead at an akinetic (scarred) segment did not adversely impact acute hemodynamic or 12-month clinical response to CRT.77
TABLE 2.1 Rates and Predictors of Acute Improvement in Left Ventricular Systolic Performance with CRT | |||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|