Dipeptidyl peptidase-4 (DPP-4) inhibitors are novel drugs for the treatment of type 2 diabetes mellitus. They exert their action through inhibition of the catabolism of locally secreted incretins such as glucagon-like peptide-4 (GLP-4) and glucose-dependent insulinotropic polypeptide (GIP) by inhibiting enzyme DPP-4. GLP-1 and GIP are secreted from the gastrointestinal tract in response to food intake. GLP-1 is secreted from L cells present in the mucosa of the small intestine and colon, whereas GIP is secreted from K cells of the jejunum. These 2 incretins lower blood glucose levels and postprandial hyperglycemia by stimulating insulin release from b cells of the pancreas, thus increasing insulin sensitivity, delaying gastrointestinal emptying, decreasing food intake through early satiety, and causing weight loss in the long term. However, their action is short-lived (2 to 3 minutes) because of catabolism by the DPP-4 enzyme. The importance of DPP-4 inhibitors lies in their blockade of the DPP-4 enzyme leading to the prevention of their catabolism and thus increasing their blood levels, extending the duration of their action, and improving their blood glucose-lowering effect. In addition to their antidiabetic action, recent experimental and clinical studies have demonstrated a pleiotropic cardiovascular protective effect of these agents independent of their antidiabetic action. They prevent atherosclerosis, improve endothelial dysfunction, lower blood pressure, and prevent myocardial injury. All these actions are discussed in this concise review. In conclusion, DPP-4 inhibitors are novel antidiabetic agents with pleiotropic cardiovascular protective effects in addition to their antidiabetic action.
Treatment of type 2 diabetes mellitus continues to evolve with the recent addition of dipeptidyl peptide-4 (DPP-4) inhibitors to the armamentarium for the treatment of type 2 diabetes mellitus. The mechanism of action of this novel class of drugs is based on their inhibitory effect of the enzyme DPP-4, which catabolizes the incretins glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). These incretins are secreted locally from the gastrointestinal tract in response to food intake. GLP-1 is secreted from L cells present in the mucosa of the distal small intestine and colon, whereas GIP is secreted from K cells of the jejunum. GLP-1 and GIP are fairly potent glucose-lowering agents, especially interfering with postprandial hyperglycemia, which has been associated with cardiovascular complications. After a meal these incretins regulate blood glucose levels by stimulating insulin release from b cells of the pancreas, increasing insulin sensitivity, delaying gastrointestinal emptying, and decreasing glucagon release from the liver. However, their action is short-lived (2 to 3 minutes) because they are rapidly catabolized by the enzyme DPP-4. Administration of DPP-4 inhibitors prevents the catabolism of GLP-1 and GIP, increases their blood levels, prolongs the duration of their action, and improves blood glucose control.
Recent studies in nonhumans and humans have shown a pleiotropic effect of these agents in addition to their antidiabetic action with respect to prevention of atherosclerosis, improvement of endothelial dysfunction, lowering blood pressure (BP), and preventing myocardial injury. The blood glucose-lowering effect of DPP-4 inhibitors is moderate, but it can be significantly increased by their combination with other antidiabetic drugs and especially metformin. In this concise review, the pleiotropic actions of DPP-4 inhibitors are discussed in the context of recent experimental and clinical evidence.
DPP-4 Inhibitors and Their Mechanism of Action
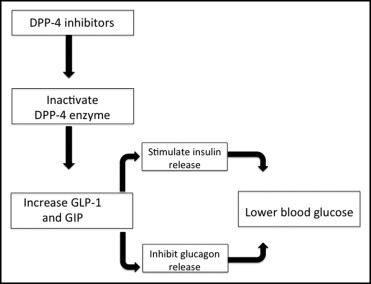
There are several DPP-4 inhibitors for the treatment of type 2 diabetes mellitus, some approved by the Food and Drug Administration and others still in development ( Table 1 ). Although they have different chemical structures, all successfully inhibit the DPP-4 enzyme and prevent the catabolism of GLP-1 and GIP. Of the 2 incretins, most of the action (80%) is mediated though GLP-1. The action of GLP-1 can be increased by the prevention of its catabolism by GLP-1 receptor stimulators such as exenatide and liraglutide, but these drugs have to be given by injection and are clinically impractical. Therefore, DPP-4 inhibitors given orally are preferable. Their mechanism of action is depicted graphically in Figure 1 . Glucoregulatory actions of GLP-1 in addition to those presented in Figure 1 include activation of insulin biosynthesis for subsequent use, b-cell proliferation and preservation, inhibition of apoptosis, delay of gastrointestinal emptying, and, of course, decreased appetite and weight loss in the long term.
| Drug | Status | Chemistry |
|---|---|---|
| Alogliptin | Pending FDA approval | Xanthine compound |
| Demogliptin | In development | Cyanopyrolidine compound |
| Linagliptin | FDA approved | Xanthine compound |
| Saxagliptin | FDA approved | Cyanopyrolidine compound |
| Sitagliptin | FDA approved | Azetidine compound |
| Vildagliptin | FDA approved | Cyanopyrolidine compound |
Cardiovascular Protective Actions of DPP-4 Inhibitors
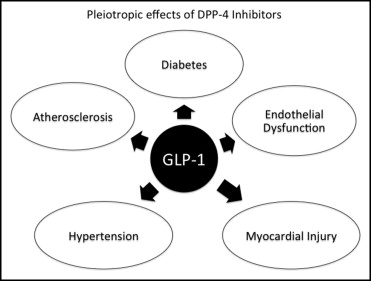
The cardiovascular protective effects of DPP-4 inhibitors are basically mediated through their control of diabetes because diabetes is a major risk factor for cardiovascular complications. However, recent experimental and clinical evidence has suggested that these agents exert additional cardioprotective effects by mechanisms independent of diabetic control. All actions of DPP-4 inhibitors are mediated through the incretins GLP-1 and GIP and especially GLP-1 as depicted in Figure 2 and discussed in the following sections.
Cardiovascular Protective Actions of DPP-4 Inhibitors
The cardiovascular protective effects of DPP-4 inhibitors are basically mediated through their control of diabetes because diabetes is a major risk factor for cardiovascular complications. However, recent experimental and clinical evidence has suggested that these agents exert additional cardioprotective effects by mechanisms independent of diabetic control. All actions of DPP-4 inhibitors are mediated through the incretins GLP-1 and GIP and especially GLP-1 as depicted in Figure 2 and discussed in the following sections.
Atherogenesis
An experimental study in knockout mice for the low-density lipoprotein receptor treated with the DPP-4 inhibitor alogliptin showed a decrease in serum lipids compared to controls. These 6-week-old mice were fed a high-fat diet or normal chow for 4 weeks and then randomized to a vehicle or alogliptin (40 mg/kg/day) for 12 weeks. Alogliptin decreased adipose tissue macrophages, inflammatory biomarkers CD11b and CD11c, aortic plaque, and aortic plaque macrophages in mice fed the high-fat and normal chow diets compared to controls. These changes occurred without weight loss, consistent with the weight neutrality of DPP-4 inhibitors as noted in clinical trials. With respect to clinical studies, in a retrospective analysis of 540,000 diabetic patients from the General Electric Centricity database, 5,861 patients were treated with the DPP-4 inhibitor sitagliptin from January 1996 to January 2008. These patients showed decreases in low-density lipoprotein cholesterol, total cholesterol, and triglycerides. Other studies in diabetic patients treated with the DPP-4 inhibitors sitagliptin and vildagliptin given in daily doses 12.5, 25, or 50 mg 2 times/day and 50 mg 2 times/day, respectively, reported decreases in total cholesterol, low-density lipoprotein cholesterol, and triglycerides and an increase in high-density lipoprotein cholesterol.
Endothelium
Emerging evidence has suggested beneficial endothelial effects of GLP-1, possibly mediated through GLP-1 receptors present in endothelial and smooth muscle cells in blood vessels. Previous animal studies have demonstrated that GLP-1 induces an endothelium-dependent vasorelaxation that is dependent on nitric oxide (NO) generation. NO is a well-known endothelium-dependent vasodilator and has been shown to improve survival of human aortic endothelial cells after ischemia–reperfusion. Also, beneficial effects on endothelial dysfunction have been observed with the GLP-1 enhancers liraglutide and sitagliptin. Liraglutide administration has been associated with inhibition of tumor necrosis factor-α, vascular cell adhesion molecule, and plasminogen activator inhibitor type 1 expression, whereas sitagliptin has been found to induce vasodilatation through NO generation. Other investigators have reported GLP-1–induced vasodilatation independent of NO generation in rat vessels.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


