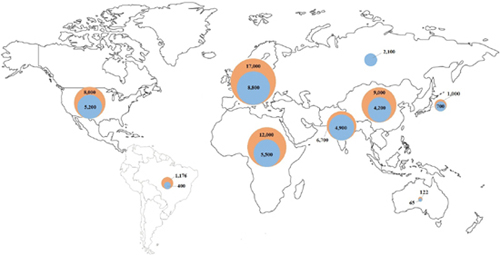
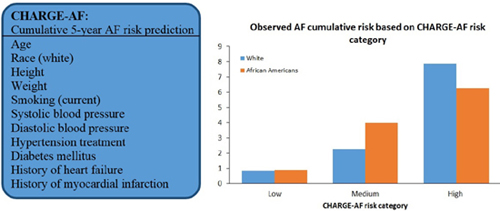
CHAPTER 1 Faisal Rahman, BMBCh, MA, and Emelia J. Benjamin, MD, ScM Atrial fibrillation (AF) is the most important clinical arrhythmia globally and affects an estimated 33 million people worldwide.1,2 Our understanding and management of AF have been evolving significantly since the first electrocardiographic AF recordings made by Einthoven3 and Lewis4 over 100 years ago. At the time, the only successful treatment was digitalis, demonstrated by Mackenzie.5 The shifting epidemiology and the increasing morbidity, mortality, social, and healthcare costs have increased the public health importance of AF in low-, middle-, and high-income countries. Providing high-quality management of AF is facilitated by an integrated approach by different members of the healthcare team. To name a few roles, team members may include an internist to assist with rate control, a primary care nurse practitioner for regular follow-up, a pharmacist for anticoagulation, and a visiting nurse to help with adherence and monitoring. AF is a supraventricular tachycardia defined by uncoordinated electrical and mechanical activity of the atria. Electrocardiographically, AF is distinguished by “irregularly irregular” RR intervals, and therefore ventricular rate, and absence of defined P waves reflecting a lack of synchronized atrial activity. The atrial electrical activity may produce fluctuations of the baseline called fibrillatory or f waves at rates over 300 beats per minute (bpm). The delay in the atrioventricular node ensures that the ventricular rate is usually significantly slower than the atrial rate in the absence of accessory atrioventricular pathways. The American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Rhythm Society (HRS)6 and European Society of Cardiology (ESC)2,7 have recommended a clinical classification system according to the duration and method of conversion of AF episodes: • Paroxysmal: AF that spontaneously terminates within 7 days, majority usually terminate within 48 hours • Persistent: AF that is present for more than 7 days • Longstanding persistent: AF present for 1 year or greater • Permanent: AF present for 1 year or greater when rhythm control is no longer planned or has failed The first-time AF is recognized regardless of symptoms, duration, or stability, it is known as the first diagnosed or detected AF. If individuals have two or more episodes, then AF is deemed as recurrent. It is not uncommon for individuals to have both paroxysmal and persistent episodes of AF, in which case the ACC/AHA/HRS and ESC guidelines recommend that the most common presentation should be used for classification.6,7 The ACC/AHA/HRS and ESC also distinguish secondary AF when an underlying acute pathology, such as myocardial infarction, myocarditis, and hyperthyroidism, accounts for the AF. Treatment of the primary process is expected to restore sinus rhythm without relapse. However, recent publications have suggested AF occurring in the context of intercurrent illnesses8 or surgery9 may be associated with long-term AF recurrence and other complications. Lone AF is sometimes used to describe AF in young individuals without secondary causes such as acute illness, hypertension, diabetes mellitus, or cardiopulmonary disease.6 The term is sometimes used to identify patients who do not need anticoagulation and are eligible for treatment with class IC antiarrhythmic agents such as flecainide. However, the use of the term has been inconsistent, and it is increasingly clear that many cases of lone AF may be associated with undiagnosed cardiopulmonary disease. Thus, current guidelines and experts discourage the use of the term “lone AF.”6,10 The ACC/AHA/HRS and ESC classification has superseded the use of the term chronic AF to describe more sustained AF.2,6 Although there are several weaknesses to the ACC/AHA/HRS and ESC schema, because of the simplicity and adoption by major cardiovascular societies, it has become widely accepted. The estimated prevalence of AF in the adult population in the United States, western Europe, and Australia is 1% to 4%,11–16 affecting an estimated 5.2 million in the United States17 and 8.8 million in the European Union18 (Figure 1.1). Over 70% of individuals with AF are over 65 years of age.11,18–20 For example, in large cross-sectional studies in the United States, the prevalence of AF was 0.1% in participants younger than 55 years,14 and over 13% in those over 80 years of age.21 However, screening studies demonstrate that AF often is unrecognized, and thus current reports likely underestimate the actual prevalence of AF.22–24 Current estimated prevalence of AF (blue), and 2050 projections worldwide.1,15,17–20,25 There is a dearth of data from low- and middle-income countries, but the available estimates report a lower prevalence (0.3% to 0.9%) in Africa and Asia compared with western Europe and the United States.19,25–29 The 2010 Global Burden of Disease study estimated the worldwide age-adjusted prevalence of AF as 596 per 100,000 men and 373 per 100,000 women, affecting approximately 33 million people.1 In 2010, the incidence rates of AF were 78 per 100,000 person-years in men and 60 per 100,000 person-years in women, increased from 61 in men and 44 in women in 1990.1 AF-associated mortality was higher in women and increased by 2-fold and 1.9-fold in men and women, respectively, from 1990 to 2010.1 There was evidence of significant regional heterogeneity in AF estimations and availability of population-based data.1 With the increasing life expectancy of the population worldwide, the burden of AF has risen in the past two decades1 and is expected to continue to rise. Projections expect the prevalence to approximately double by 2050 in many nations.11,17,18,20,30 The Framingham Heart31 and Rotterdam studies32 evaluated the lifetime risk of AF in individuals of European ancestry. In the Rotterdam Study,32 the lifetime risk of AF at 55 years of age was 22% to 24%, and in the Framingham cohort31 the lifetime risk at 40 years of age was 23% to 26%. Because of the increase in the prevalence of AF with age, the lifetime risk at 70 years of age remains high at 21% to 24%.31 Aside from advancing age, there is a wide variety of other risk factors associated with AF. The most prevalent risk factors are hypertension and elevated body mass index. In the Atherosclerosis Risk in Communities (ARIC) study, the population-attributable risk for AF was 21.6% for elevated blood pressure and 17.9% for overweight or obesity.33 Other important predictors of AF in both males and females include diabetes mellitus, smoking, myocardial infarction, valvular heart disease,34 heart failure,34,35 hyperthyroidism,36 and atrial structural abnormalities.37 Before widespread early treatment of streptococcal infections, rheumatic heart disease and mitral valve disease were important risk factors for AF in the United States and Western Europe.38 However, in low- and middle-income countries rheumatic heart disease remains a major risk factor for AF,19,39 and the prevalence is over 60% among individuals with AF in some countries.39,40 Over the past decade, data have demonstrated that AF can be inherited.41–44 In the Framingham Heart Study, individuals with a first-degree relative with AF had a higher risk of new-onset AF compared with individuals without a family history (hazard ratio 1.4).42 Furthermore, the risk of early onset AF (less than 6044 or 6542 years of age) was three to five times higher in individuals with a family history of early onset AF. Genome-wide association studies have demonstrated significant associations between AF and three major genetic loci: 4q25 (near PITX2 transcription factor important in cardiac development),45–47 16q22 (ZFHX3, unclear role but may be important in interstitial matrix deposition)48–50 and 1q21 (KCNN3, a calcium-activated small conductance potassium channel).51,52 Although initial studies identified the three loci in whites, more recent studies support their role in individuals of non-European ancestry.46,50,51,53 A recent meta-analysis identified six more genetic loci associated with AF,54 and undoubtedly other predisposing genetic variants will be identified in the future.55 The precise causal variants and mechanisms underlying the identified genetic polymorphisms remain unclear. However, they likely represent new pathways in AF pathophysiology, which may lead to future etiologic insights and therapeutic advances. Risk factors for AF, including age, race, blood pressure, diabetes mellitus, smoking, heart failure, myocardial infarction, recently have been incorporated into risk prediction tools that can help identify high-risk individuals.34,35,56 The CHARGE-AF risk prediction model (Figure 1.2) was developed using data from the Framingham Heart Study, Cardiovascular Heart Study, and the Atherosclerosis Risk in Communities; and validated in two European cohorts, Age, Gene and Environment – Reykjavik Study and Rotterdam Study.34 In the future, the CHARGE-AF model may help multidisciplinary healthcare teams identify high-risk individuals to target AF screening and primary prevention strategies.57 In addition, risk-prediction models may serve as benchmarks to evaluate novel putative risk markers for AF. Variables in the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) AF Trial 5-year AF risk predictive model (box). Cumulative risk of AF based on predicted risk category according to CHARGE-AF among whites and African Americans. (Source: Graphs modified from Alonso et al.34) The most feared complication of AF is embolic stroke, with a 4.5% average annual risk in individuals with AF.58,59 The attributable risk of AF for ischemic stroke is 3% to 8% in individuals 60 to 69 years of age but over 20% in individuals 80 to 89 years old.60,61 AF is also associated with an increased risk of cognitive impairment,62 chronic kidney disease,63 myocardial infarction,64 and heart failure.65 Additionally, atrial contraction contributes approximately 20% or more to the cardiac output,66 and patients with heart failure may experience acute decompensation with the development of AF.67,68 The mortality risk is approximately doubled by the presence of AF compared with individuals without AF.42,69 The 2010 Global Burden of Disease study estimated age-adjusted disability-associated life-years caused by AF at 64.5 and 45.9 per 100,000 men and women, respectively.1 The high prevalence and resulting complications of AF account for significant financial costs to society. In the United States, the estimated annual incremental cost of AF is $26 billion,70 and €6.2 billion in Spain, Greece, Italy, Spain, and the Netherlands combined.71 In addition, AF accounts for 3.2 million extra hospital days in the United States,70 as well as loss of work days,71 and impaired quality of life.72 Estimations of financial costs in low- and middle-income countries are lacking. A significant percentage of AF recurs and progresses from paroxysmal to persistent and permanent AF in longitudinal studies.42,73–76 After 12 months follow-up in the Euro Heart Survey, 15% of individuals with paroxysmal AF progressed to persistent AF,73 and in a US cohort 18.6% of individuals progressed from paroxysmal to persistent or persistent to permanent AF.77 However, in the Framingham Heart Study, among first-detected AF approximately 10% of participants did not have a documented recurrence within 2 years.42 The longitudinal history of AF remains incompletely defined, as studies have demonstrated that progression to other stages may remain unrecognized.23,74 Predictors of the progression of AF include advancing age,73,74,77 increasing body mass index,76 hypertension,73 heart failure,73,74 valvular heart disease,78 and history of stroke or transient ischemic attack.73,77 The development of AF is thought to occur through ectopic focal triggered or reentry mechanisms.6,79,80 A focal source appears to most commonly originate at the pulmonary veins in the left atrium. Pulmonary vein isolation is the most common strategy used to treat AF.79,81–83 Factors that promote AF in the pulmonary veins include short duration of the action potentials, lower resting membrane potentials, and lack of uniform alignment of myofibrils.80,84,85 However, focal AF may also originate outside of the pulmonary veins.86,87 Many mechanisms have been proposed to explain what sustains AF. They include the multiple reentrant wavelet hypothesis (random reentry),88–90 ordered reentry of short cycle length (e.g., head–tail interaction),91,92 and one or more foci firing rapidly.93 The mechanisms have not been fully elucidated, which has contributed to the incomplete success of AF ablation to date. Electrophysiology studies have demonstrated that electrical heterogeneity exists within the atria that provide the foundation for the mechanisms discussed previously.94,95 AF is associated with changes in modulation of Ca2+ and K+, which affect the resting membrane potential, refractory period, and action potential duration in the atria, providing the substrate for AF.96–99 Animal and human studies demonstrate a reduction in the L-type Ca2+ current and therefore calcium-induced sarcoplasmic Ca2+ release, shortening the action potential duration.100,101 Similarly, changes in potassium channels are found in AF.102–104 However, it is not clear if these changes precede the onset of AF in humans. AF results in significant changes in the expression and function of multiple genes, including ion channels, structural proteins, and the activation of the renin–angiotensin–aldosterone system.105,106 Alterations in gene expression contribute to electrical and structural remodeling, which perpetuate AF and appear to promote the evolution of paroxysmal to persistent and permanent AF.106,107 Alterations in gene expression may provide novel future targets for the treatment of AF. Several important atrial structural changes are associated with the initiation and maintenance of AF, including fibrosis, dilatation, apoptosis, and hypertrophy. AF itself is associated with structural remodeling that promotes the maintenance and possible progression of AF from paroxysmal to persistent and permanent.108–112 In postmortem studies, patients with AF had more extensive atrial fibrosis compared with those without AF.111 Furthermore, atrial fibrosis was greater in individuals with persistent and permanent AF compared with paroxysmal AF.111,113 Increased left atrial size has long been established as a risk factor for the development37,114,115 and recurrence of AF.112,116,117 The presence of AF itself appears to cause left atrial dilatation, thereby promoting its recurrence.110,118
Classification and Epidemiology of Atrial Fibrillation
INTRODUCTION
CLASSIFICATION
EPIDEMIOLOGY
Risk Factors
Risk Prediction
Complications and Costs
LONGITUDINAL HISTORY
PATHOPHYSIOLOGY
Electrical Mechanisms
Atrial Structural Changes
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


