Chylothorax and Pseudochylothorax
At times, pleural fluid is milky or at least turbid. When the milkiness or turbidity persists after centrifugation, it is almost always due to a high lipid content of the pleural fluid. High levels of lipid accumulate in the pleural fluid in two situations. First, when the thoracic duct is disrupted, chyle can enter the pleural space to produce a chylous pleural effusion. In this situation, the patient is said to have a chylothorax (1). Second, in long-standing pleural effusions, large amounts of cholesterol or lecithin-globulin complexes can accumulate in the pleural fluid to produce a chyliform pleural effusion. The patient is then said to have a pseudochylothorax. It is important to differentiate these two conditions because their prognosis and management are completely different.
CHYLOTHORAX
A chylothorax is formed when the thoracic duct is disrupted and chyle enters the pleural space.
Pathophysiologic Features
Dietary fats in the form of long-chain triglycerides are transformed into chylomicra and very-low-density lipoproteins. These are secreted into the intestinal lacteals and lymphatics and are then conveyed to the cisterna chyli, which overlies the anterior surface of the second lumbar vertebra, posterior to and to the right of the aorta. Usually, one major lymphatic vessel, the thoracic duct, leaves the cisterna chyli and passes through the esophageal hiatus of the diaphragm into the thoracic cavity. The thoracic duct ascends extrapleurally in the posterior mediastinum along the right side of the anterior surface of the vertebral column and lies between the azygos vein and the descending aorta in close proximity to the esophagus and the pericardium. At the level of the fourth to sixth thoracic vertebrae, the duct crosses to the left of the vertebral column and continues cephalad to enter the superior mediastinum between the aortic arch and the subclavian artery and the left side of the esophagus.
Once the thoracic duct passes the thoracic inlet, it arches 3 to 5 cm above the clavicle and passes anterior to the subclavian artery, vertebral artery, and thyrocervical trunk to terminate in the region of the left jugular and subclavian veins. Wide anatomic variations may exist in all portions of the thoracic duct. More than one thoracic duct may leave the cisterna chyli. The duct may continue on the right side of the vertebral column to enter the veins in the right subclavian region. Multiple anastomoses usually exist between various lymphatic channels, and direct lymphaticovenous communications with the azygos vein may be present (2,3).
The drainage from the thoracic duct is called chyle. Chyle appears grossly as a milky, opalescent fluid that usually separates into three layers upon standing: a creamy uppermost layer containing chylomicrons, a milky intermediate layer, and a dependent layer containing cellular elements, most of which are small lymphocytes (4). If the patient has not eaten, however, chyle may be only slightly turbid because its lipid content will be reduced. Chyle is bacteriostatic and does not become infected even when it stands at room temperature for several weeks (5). Lampson (5) reported that Escherichia coli and Staphylococcus aureus were unable to grow in 100% chyle. Chyle that is extravasated into the pleural cavity is not irritating and usually does not evoke the formation of a pleural peel or a fibroelastic membrane.
Each day, between 1,500 and 2,500 mL of chyle normally empties into the venous system (6). The ingestion of fat can increase the flow of lymph in the thoracic duct by 2 to 10 times the resting level for several hours (7). Ingestion of liquid also increases the chyle flow, whereas the ingestion of protein or carbohydrates has little effect on the lymph flow (7). The protein content of chyle is usually above 3 g/dL, and the electrolyte composition of chyle is similar to that of serum (7).
The primary cell in chyle is the small lymphocyte, and lymphocyte counts range from 400 to 6,800/mm3 (4). Prolonged drainage of a chylous pleural effusion can result in profound T-lymphocyte depletion. Breaux and Marks (8) reported that the total peripheral lymphocyte count fell from 1,665 to 264/mm3 with 14 days of chest tube drainage in one patient with a chylothorax. Almost all the lymphocytes in the pleural fluid were T lymphocytes.
A chylothorax results when the lymphatic duct becomes disrupted. Ligation of the thoracic duct at any point in its course does not produce a chylothorax in experimental animals (6), presumably as a result of the many collateral vessels and lymphaticovenous anastomoses. Ligation of the superior vena cava produces chylothorax approximately half the time in experimental animals. In the experimental animal, laceration or transection of the thoracic duct does not always produce a chylothorax. Hodges et al. (9) produced a 2.5-cm longitudinal laceration of the thoracic duct in three dogs at the level of T9 and transected the thoracic duct at this level in three other dogs. They reported that all animals developed a pleural effusion but that the effusion ceased to form after 2 to 5 days in the animals with lacerations and after 4 to 10 days in the animals with transections (9). Lymphangiograms demonstrated that there was no continuity of the thoracic duct in animals with transections and researchers concluded that lymph was being conveyed by collaterals (9).
Etiologic Factors
The causes of 143 chylothoraces from five separate earlier series (6,7,10,11,12) are tabulated in Table 26.1. The causes of 203 chylothoraces seen at the Mayo Clinic between January 1980 and December 2000 were as follows: surgery or trauma, 101 patients (49.8%); malignancy, 34 (16.7%); congenital or acquired lymphatic disorders, 19 (9.4%); chylous ascites, 16 (7.9%); miscellaneous medical causes, 20 (10%); and no identifiable cause, 13 (6.4%) (13). The differences in the distribution of the diagnoses in this latter study compared with the former studies may be due in part to the fact that the Mayo Clinic is a tertiary referral center and that the distribution of diagnoses is changing with time.
TABLE 26.1 ▪ Causes of 143 Chylothoraces from Five Separate Series | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
For convenience, the causes of chylothorax can be grouped into four different categories, namely, trauma, tumor, miscellaneous, and idiopathic (14). Trauma is the leading cause of chylothorax. This trauma is usually a cardiovascular, pulmonary, or esophageal surgical procedure. Chylothorax appears particularly frequently following operations in which the left subclavian artery is mobilized (15). The incidence of chylothorax after most thoracic surgeries is less than 1.0%. The incidence of chylothorax was 0.5% in one series of 2,660 cardiovascular operations (16), whereas it was 2.4% in a series of 1,110 lobectomies and pneumonectomies with systematic mediastinal lymph node dissection (17). The incidence of chylothorax is relatively high in children undergoing cardiothoracic surgery. In one series of 1,257 surgeries, there were 48 cases of chylothorax (3.8%) (18). The incidence exceeded 10% in patients undergoing the Fontan procedure, heart transplantation, and repair of the Tetralogy of Fallot (18). The incidence of chylothorax following esophageal resection is relatively high; it was 3.8% in one series of 892 cases (19). Dougenis et al. (20) reported that the incidence of chylothorax following esophageal surgery was significantly higher when the main thoracic duct was not ligated at the time of the resection. A subsequent study (21) randomized 653 patients undergoing esophageal resection to undergo or not undergo thoracic duct ligation. They reported that the incidence of chylothorax in the treatment group (0.3%) was significantly less than in the control group (2.1%) (21).
Chylothorax has also been reported as a complication of coronary artery bypass surgery when the internal mammary artery is harvested (22), heart transplant (23), high translumbar aortography (24), sclerotherapy for esophageal varices (25), thoracolumbar fusion for correction of kyphosis (26), and cervical node dissection (27).
Chylothorax has also been reported as a complication of coronary artery bypass surgery when the internal mammary artery is harvested (22), heart transplant (23), high translumbar aortography (24), sclerotherapy for esophageal varices (25), thoracolumbar fusion for correction of kyphosis (26), and cervical node dissection (27).
Of course, penetrating trauma to the chest or neck such as gunshot or knife wounds can also sever the thoracic duct and may lead to chylothorax. Trauma in which the spine is hyperextended or a vertebra is fractured is most likely to cause chylothorax, particularly if the injury occurs after the recent ingestion of a fatty meal (27). A chylothorax secondary to closed trauma is usually on the right side, and the site of rupture is most commonly in the region of the 9th or 10th thoracic vertebra (27). Such trauma includes falls from a height, motor vehicle accidents, compression injuries to the trunk, heavy blows to the back or stomach, and childbirth (28). The injury may be less impressive, and chylothoraces have been attributed to coughing, vomiting, and weight lifting. In one well-documented case report, an episode of vigorous stretching while yawning was followed by swelling in the left supraclavicular fossa and the development of bilateral chylothoraces (29).
Another leading cause of chylothorax is malignancy. The most common malignancy to cause a chylothorax is a lymphoma, and lymphomas accounted for 75% of the chylothoraces due to malignancies are listed in Table 26.1. In the series from the Mayo Clinic (13), lymphomas (20 non-Hodgkin lymphoma and 3 Hodgkin disease) accounted for 68% of the chylothoraces due to malignancy. Other malignancies producing a chylothorax in the Mayo Clinic series included chronic lymphocytic leukemia 5, metastatic disease 5, and lung cancer 1 (13). Chylothorax may be the presenting symptom of lymphoma (7,11,15). Therefore, a nontraumatic chylothorax is an indication for a diligent search for a lymphoma. In the series of Roy et al. (11) before the availability of computed tomographic (CT) scans, the diagnosis of lymphoma was not established until 6 to 12 months after the appearance of the chylothorax in four patients.
The third category of chylothorax is the miscellaneous category. Thrombosis of the superior vena cava or the subclavian vein is becoming one of the more common causes of chylothorax. Berman et al. (30) reviewed the case histories of 37 infants and children with thrombosis of their superior vena cava in a newborn and pediatric intensive care unit and reported that 9 (24%) had a chylothorax. We reviewed cases of the superior vena caval syndrome at my previous hospital over a 6-year period and found that chylothorax complicated 4 of 76 cases (31). Chylothorax can also complicate innominate vein (32) or left subclavian vein thrombosis (33). Cirrhosis is a relatively common cause of chylothorax. Romero et al. (34) analyzed 24 cases of chylothorax occurring at their institution and reported that 5 (21%) were secondary to cirrhosis. Interestingly, the mean protein level in these five chylothoraces was only 1.7 g/dL (compared with 4.1 g/dL in the other chylous effusions), the mean lactate dehydrogenase (LDH) level was only 96 IU/L (compared with 351 IU/L in the other chylous effusions) and ascites was present in three of the five patients (34). On rare occasions, a chylothorax is associated with heart failure or the nephrotic syndrome and the effusion is also a transudate in these instances (35). In most patients with the nephrotic syndrome and a chylothorax, the chylothorax is secondary to chylous ascites, but on occasions, it can be secondary to superior vena caval thrombosis (36).
Many other causes of chylothorax have been reported, but even when all are grouped together, they account for only a small percentage of chylothoraces. The most interesting of these is pulmonary lymphangioleiomyomatosis (LAM), which has associated interstitial parenchymal infiltrates, and is discussed later in this chapter. Other causes include Gorham syndrome (37) (also discussed later in this chapter), Kaposi sarcoma in patients with acquired immunodeficiency syndrome (AIDS) (38,39), the yellow nail syndrome (14), filariasis, paragonimiasis (40), giant lymph node hyperplasia (Castleman disease) (41), lymphangiomatosis (42), familial lymphedema (43), lymphangitis of the thoracic duct, obstruction of the superior vena cava secondary to Behçet syndrome (44,45), tuberculosis (46), sarcoidosis involving the intrathoracic lymph nodes (47), aneurysms of the thoracic aorta that erode the duct, abnormalities of the lymphatic vessels such as intestinal lymphangiectasis (48) or reticular hyperplasia (12,49), radiation-induced mediastinal fibrosis (50), and hypothyroidism (51).
The fourth category of chylothorax is idiopathic, including most cases of congenital chylothorax. One should exclude lymphoma as a cause of the chylothorax before it is labeled idiopathic. Most cases of idiopathic chylothorax in the adult are probably due to minor trauma, such as coughing or hiccupping, after the ingestion of fatty meals.
Chylothorax is the most common form of pleural effusion encountered in the first few days of life (52). The fetal pleural effusion discussed in Chapter 20 is probably also a chylothorax. Neonatal chylothorax is relatively uncommon; during a 22-year period, 12 cases were diagnosed at the Hospital for Sick Children, which is a large pediatric tertiary care center (53). The babies are usually born at full term after normal labor and delivery. The etiology of congenital chylothorax is unknown (54). Abnormalities of the thoracic duct have not been found in most babies who have undergone exploratory thoracotomy (52). Several cases of generalized pleural oozing have been described during surgery (53). It is possible that birth trauma may result in a tear of a major lymphatic channel in at least some individuals. Most neonatal chylothoraces represent pleural effusions that had been present antenatally. In some cases, a congenital chylothorax is associated with Turner’s syndrome, Noonan’s syndrome, or Down’s syndrome (54). Congenital chylothorax is also more common in infants who are hydropic or who have polyhydramnios (53).
Interestingly, mice that lack the integrin α9 β1 appear normal at birth but then develop respiratory failure and die between 6 and 12 days of age (55). The respiratory failure is caused by bilateral chylothoraces. Although the thoracic duct appears normal grossly, microscopic examination reveals edema and lymphocytic infiltration in the chest wall (55). Members of the integrin family of adhesion receptors mediate both cell-cell and cell-matrix interactions and have been shown to play vital roles in embryonic development, wound healing, and other biologic processes. It has been postulated that integrin α9 β1 deficiency could be one cause of congenital chylothorax (55).
Clinical Manifestations
The initial symptoms of chylothorax are usually related to the presence of the space-occupying fluid in the thoracic cavity, and, therefore, patients have dyspnea. On rare occasions, the patient can develop a tension chylothorax with compromise of the systemic circulation (56). Pleuritic chest pain and fever are rare because chyle is not irritating to the pleural surface. With traumatic chylothorax, a latent period of 2 to 10 days usually occurs between the trauma and the onset of the pleural effusion (27). There is one case report in which the latent period was 11 weeks (57). Lymph collects extrapleurally in the mediastinum after the initial thoracic duct disruption, forms a chyloma, and produces a posterior mediastinal mass (3). The mediastinal pleura eventually ruptures, chyle gains access to the pleural space, and dyspnea is produced by the chyle compressing the lung. At times, hypotension, cyanosis, and extreme dyspnea occur when the chyloma ruptures into the pleural space. The ruptured chyloma is no longer visible radiographically.
With nontraumatic chylothorax, the onset of symptoms is usually gradual. In congenital chylothorax, the infant develops respiratory distress in the first few days of life; 50% of patients have symptoms within the first 24 hours, whereas 75% have symptoms by the end of the first week (52). The chyle production in a neonate may exceed 250 mL/day (54).
The main threat to life from chylothorax is malnutrition and a compromised immunologic status. Because the thoracic duct carries 2,500 mL of fluid daily that contains substantial amounts of protein, fats, electrolytes, and lymphocytes, the patient can become cachectic rapidly if this amount of chyle is removed daily through chest tubes or repeated thoracentesis. In addition, the patients develop lymphopenia and a compromised immunologic status because of the removal of large numbers of lymphocytes with the chyle (58). In one case report, one patient had more than 35 L of fluid withdrawn over a 14-day period that contained 2.3 kg of fat and 0.7 kg of protein. During this period, the peripheral lymphocyte count dropped from 1,665 to 264/mm3 (8). Indeed, until Lampson initially described successful ligation of the thoracic duct in 1948 (5), the mortality rate from chylothorax was 50%. When managing a patient with chylothorax, one should abandon conservative treatment before the patient becomes too malnourished and immunocompromised.
Diagnosis
The diagnosis of chylothorax is usually not difficult because chyle typically has a distinctive white, odorless, milky appearance. When such fluid is found, the main differentiation is between chylothorax, empyema, and a pseudochylothorax. The milkiness with empyema is caused by the suspended white blood cells (WBCs) and debris, and if such fluid is centrifuged, the supernatant is clear. The cloudiness of the chyliform pleural effusion from a pseudochylothorax is also caused by high lipid levels and either cholesterol or lecithinglobulin complexes. Chylous and chyliform pleural fluids remain opaque after centrifugation.
If cholesterol crystals are responsible for the turbidity, they may be easily demonstrated by examination of the pleural fluid sediment (Fig. 26.1). If the turbidity is
due to high levels of cholesterol, the turbidity will clear when 1 to 2 mL of ethyl ether is added to a test tube containing the fluid; if the turbidity is due to chylomicrons or lecithin complexes, the turbidity does not clear (59).
due to high levels of cholesterol, the turbidity will clear when 1 to 2 mL of ethyl ether is added to a test tube containing the fluid; if the turbidity is due to chylomicrons or lecithin complexes, the turbidity does not clear (59).
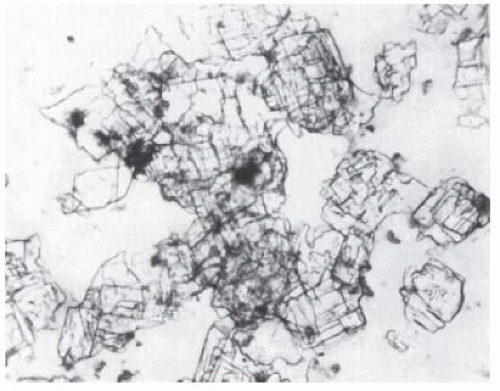 FIGURE 26.1 ▪ Cholesterol crystals. Typical large polyhedric crystals from a patient with a cholesterol pleural effusion. This patient had a rheumatoid pleural effusion. |
Not all chylous pleural effusions have the typical, milky appearance. With congenital chylothorax, the pleural fluid is initially serous and turns chylous only when milk feedings are started (52). Because congenital chylothorax is the most common cause of pleural effusion in the newborn (52), pleural fluid triglyceride and lipoprotein analyses should be performed in all newborns with pleural effusion. In adults, the pleural fluid does not always look like typical chyle. Maldonado et al. (60) reported that the pleural fluid appeared milky in only 44% of 74 chylothoraces, whereas Romero et al. (34) reported that 10 of 24 (42%) patients with chylothorax had nonmilky pleural fluid. In this study of 809 patients with pleural effusions, 24 (3%) had chylothorax (34). This study suggests that lipid measurements might be indicated in all patients with pleural effusions of unknown etiology in order to rule out the diagnosis of chylothorax.
The pleural fluid with chylothorax usually meets exudative criteria. However, Diaz-Guzman et al. (61) summarized the world’s literature on chylothoraces that met transudative criteria. They were able to find 15 such instances and 14 were due to cirrhosis, nephrosis, or congestive heart failure.
Triglyceride Measurement
The best way to establish the diagnosis of chylothorax is by measuring the triglyceride and cholesterol levels in the pleural fluid (Fig. 7.8). If the pleural fluid triglyceride level is above 110 mg/dL and the ratio of the pleural fluid to serum cholesterol is less than 1.0, the diagnosis of chylothorax is established. The cholesterol ratio is used to exclude pseudochylothorax because some patients with chyliform pleural effusions also have triglyceride levels above 110 mg/dL, but their pleural fluid to serum cholesterol ratio will exceed 1.0 (34). Although it has been suggested that to establish the diagnosis of chylothorax the pleural fluid triglyceride level should be more than the serum triglyceride level (34), this criterion appears to be unnecessary because there is no relationship between the pleural fluid and the serum triglyceride levels in patients not having a chylothorax (62). The only other situation in which the pleural fluid triglyceride is above 110 mg/dL is when intravenous fluid containing high levels of triglycerides leaks from a central vein into the pleural space (63). One should usually be able to differentiate chylothorax and pseudochylothorax by the clinical course. A chylothorax has an acute onset with normal pleural surfaces, whereas a pseudochylothorax occurs in a patient with a longstanding pleural effusion with thickened pleura (64). If any doubt exists, the pleural fluid should be analyzed for chylomicrons by lipoprotein analysis. The demonstration of chylomicrons in the pleural fluid by lipoprotein analysis establishes the diagnosis of chylothorax (10).
Lipophilic Dye Ingestion
Another test for the diagnosis of chylothorax is ingestion of a fatty meal with a lipophilic dye, followed by a thoracentesis 30 to 60 minutes later, to ascertain whether the pleural fluid has changed in color (65). The most commonly used dye is Drug and Cosmetic Green No. 6, a coal-tar dye. One gram of this dye is mixed thoroughly with 0.25 lb of butter, and the mixture is spread on a slice of bread. The patient eats the bread, and a thoracentesis performed 30 to 60 minutes later should yield green fluid if a chylothorax is present. I have attempted this test in approximately six different patients. The greatest problem I encountered was in maintaining a straight face when I asked the patient to eat the disgusting green mess. Because the diagnosis is usually easily established with triglyceride and lipoprotein analysis, I no longer ask my patients with suspected chylothorax to eat dark green sandwiches.
Imaging of the thoracic duct
At times, one would like to demonstrate the location of the disruption of the thoracic duct. In other
patients, visualization of the thoracic duct is desired in patients with chylothorax for which there is no ready explanation. In the past, this has most commonly been done by injecting a dye in the dorsum of the feet, which is subsequently absorbed in the lymphatics. When the dye is conveyed to the chest, the thoracic duct can be visualized. However, most medical centers no longer perform lymphangiograms.
patients, visualization of the thoracic duct is desired in patients with chylothorax for which there is no ready explanation. In the past, this has most commonly been done by injecting a dye in the dorsum of the feet, which is subsequently absorbed in the lymphatics. When the dye is conveyed to the chest, the thoracic duct can be visualized. However, most medical centers no longer perform lymphangiograms.
There have been two recent reports that have demonstrated that imaging of the thoracic duct can be performed by lymphoscintigraphy (66,67). With this procedure, Technetium-99m human serum albumin is injected into the dorsum of the foot or hand and subsequently the thoracic duct is imaged in nuclear medicine. There is one paper that reported the successful imaging of the thoracic duct after the oral ingestion of iodine-123-labeled 15-(4-iodophenyl)-3-(R,S)-methyl-pentadecanoic acid (I-123 BMIPP) (68). The thoracic duct can be imaged approximately 80 minutes after the ingestion of this tracer (68). The advantage of this procedure is that it does not require the difficult injections into the foot.
Treatment
The main danger to patients with chylothorax is that they become malnourished and immunocompromised because of the removal of large amounts of protein, fat, electrolytes, and lymphocytes from the body with repeated thoracentesis or chest tube drainage. In the past, the mortality rate from chylothorax approached 50%. When managing a patient with chylothorax, one must treat the chylothorax definitively, such as with thoracic duct ligation or pleuroperitoneal shunt implantation, before the patient becomes too cachectic to tolerate the operation. Because the management of chylothorax differs for traumatic, nontraumatic, and congenital chylothoraces, treatment regimens are described separately.
Traumatic Chylothorax
The general aims in treating the patient with traumatic chylothorax are relief of dyspnea by removal of the chyle, prevention of dehydration, maintenance of nutrition, and a reduction in the rate of chyle formation. When a postoperative chylothorax is discovered, tube thoracostomy should be performed to remove the chyle and relieve the dyspnea. In this situation, consideration should be given to recycling the chyle to prevent malnutrition and immunosuppression. Thomson and Simms (69) reported one case in which the chyle was reinfused directly from the chest tube into the subclavian vein for a total of 18 days. There have been no large series evaluating this procedure. In the first half of the last century, a couple of patients died from “anaphylaxis” soon after chyle infusion was started (69).
When a postoperative chylothorax is managed initially with tube thoracostomy, efforts should be made to decrease the flow of chyle through dietary manipulation. The flow of chyle is minimized if all nourishment by mouth is halted and if the patient’s gastrointestinal tract is maintained as empty as possible by constant gastric suction (70). The patient’s nutritional status can be maintained with intravenous hyperalimentation (71). In the past, attempts have been made to decrease the lymph flow by providing the fat calories in the diet with medium-chain triglycerides (71). These triglycerides have 10 or fewer carbon atoms and are absorbed directly into the portal vein and thereby gain entrance to the circulatory system without ever entering the thoracic duct (72). Because they are relatively unpalatable and hyperalimentation decreases the flow of chyle much more, hyperalimentation rather than medium-chain triglycerides is recommended when one wishes to reduce the flow of chyle. The flow of chyle is also decreased if the patient stays in bed because any lower extremity movement increases the flow of lymph (72). There is one report in which the flow of chyle was reduced markedly with inhaled nitrous oxide in a 41-week-old infant with a postoperative chylothorax (73). The reduction in the flow of chyle was thought to be due to alleviation of the central venous hypertension (73). One must question the efficacy of this approach as there have been no subsequent articles on this subject.
The defect in the thoracic duct frequently closes spontaneously in traumatic chylothorax. If the thoracic duct is transected in dogs, chyle ceases to enter the pleural space within 10 days as collateral lymphatic channels are formed (9). In one series of 22 children with postoperative chylothorax, 19 (86.4%) closed spontaneously when the patients were treated concomitantly with total parenteral nutrition or low-fat enteral diets (74). The average duration of drainage of these 19 patients was 13.7 days, with a range of 7 to 30 days (74). In another series of 47 adults with postoperative chylothorax from the Mayo Clinic (75), the leak closed spontaneously in 7 of 36 patients (19%) who received central hyperalimentation and in 6 of 11 patients (55%) who were treated with mediumchain triglyceride diets (75).
Chylothoraces that occur after pulmonary resections are usually small and resolve with medical
therapy (76). In one series, 20 of 26 cases resolved with only medical therapy (76). The probable explanation for these excellent results is that the injury is usually to a tributary of the thoracic duct and not to the thoracic duct itself (17,76).
therapy (76). In one series, 20 of 26 cases resolved with only medical therapy (76). The probable explanation for these excellent results is that the injury is usually to a tributary of the thoracic duct and not to the thoracic duct itself (17,76).
If large amounts of chyle continue to drain for more than several days postoperatively, a procedure should be performed to treat the chylothorax definitively. The alternatives at this juncture are (a) to administer somatostatin or its analogue octreotide, (b) to insert a pleuroperitoneal shunt, (c) to percutaneously embolize the thoracic duct using a transabdominal approach, (d) to attempt to create a pleurodesis to obliterate the pleural space through tube thoracostomy, (e) to perform thoracoscopy with pleural abrasion or partial pleurectomy to create a pleurodesis, (f) to perform thoracoscopy with attempted ligation of the thoracic duct, or (g) to perform a thoracotomy with ligation of the thoracic duct.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


