Chronic venous disorders are among the most common afflictions of Western populations affecting 50% to 85% of individuals.
1 It is estimated that as many as 164 of every 1000 people seek medical advice for venous problems.
2 Chronic venous
disorders include the full spectrum of venous problems, ranging from telangiectasias to venous ulcers, while the term chronic venous
disease refers to those abnormalities with signs and symptoms warranting further investigation.
1 Chronic venous
insufficiency specifically refers to advanced manifestations of disease associated with ambulatory venous hypertension including edema, skin changes, and ulceration.
Varicose veins are the most common manifestation of primary chronic venous disease and are defined as dilated, palpable, tortuous veins greater than 3 mm in diameter that do not discolor the overlying skin.
3 In contrast, reticular veins are dilated but nonpalpable blue dermal veins less than 3 mm in diameter and are distinguished from smaller red to purple telangiectasias that are usually less than 1 mm in diameter. The major saphenous trunks are not usually abnormal in patients with telangiectasias and reticular veins but are often incompetent with varicose veins.
Long-standing ambulatory venous hypertension leads to more advanced manifestations of venous disease, including skin changes and ulceration. Hyperpigmentation, most often in a gaiter distribution extending from just below the malleoli to the posterior prominence of the calf muscle, results from the extravasation of red blood cells with subsequent accumulation of hemosiderin within dermal macrophages.
3,
4 The subcutaneous tissue later becomes fibrotic and may be associated with cutaneous weeping, scaling, and erythema characteristic of venous eczema. Lipodermatosclerosis refers to the subcutaneous fibrosis and chronic inflammation that result from sustained venous hypertension. Ulceration is the most advanced stage of chronic venous disease.
Chronic venous disorders require a uniform system for describing the clinical manifestations and associated pathologic features, and the CEAP classification has become the international standard.
3,
5 As summarized in
Table 21.1, the CEAP system includes descriptions of the clinical disease class (C) based upon objective signs; the underlying etiology (E); the anatomic (A) distribution of reflux and obstruction in the superficial, deep, and perforating veins; and the underlying pathophysiology (P). Seven clinical disease categories are recognized including asymptomatic limbs (C
0) and those with telangiectasias (C
1), varicose veins (C
2), edema (C
3), skin changes without ulceration (C
4a and C
4b), healed ulcers (C
5), and active ulcers (C
6). Etiology is categorized as being congenital (E
c), primary (E
p), or secondary (E
s), while the pathophysiology is defined as being secondary to reflux (P
r), obstruction (P
O), or both (P
R+O).
Primary venous disorders are associated either with degenerative changes in the vein wall and valves, resulting in valvular incompetence, or with compressive lesions, most often in the pelvis, leading to venous obstruction. In contrast, secondary venous disorders most often result from a preceding deep venous thrombosis (DVT). When developing after an episode of DVT, manifestations of pain, edema, skin changes, and ulceration are commonly referred to as the postthrombotic syndrome.
Venous claudication, primarily due to iliofemoral venous obstruction, is not included in the CEAP clinical classification and warrants special consideration. Patients with symptoms of venous claudication complain of lower extremity tightness and bursting pain with vigorous exercise.
6 In contrast to arterial claudication, the pain is often less severe and affects the entire limb rather than specific muscle groups; its onset is more gradual and occurs with a higher intensity of activity, and it may require 15 to 20 minutes of rest, frequently with leg elevation, for relief. Associated findings may include swelling of the thigh and calf, cyanosis, and prominence of the superficial veins. Patients may also have other manifestations of chronic venous disease, including varicose veins and ulceration.
7It should be clear that the appropriate description and management of chronic venous disorders usually requires more than can be determined from the history and physical examination alone. Precise definition of the underlying etiology, anatomy, and pathophysiology usually requires additional diagnostic studies.
THE DIAGNOSTIC APPROACH TO CHRONIC VENOUS DISORDERS
The manifestations of chronic venous disease may result from either venous reflux or obstruction. Venous reflux is defined as retrograde flow of abnormal duration and may be either axial or segmental.
1 Axial reflux refers to uninterrupted retrograde venous flow from the groin to the calf and may occur through a combination of superficial, deep, and perforator pathways. As discussed below, hemodynamically significant obstruction is difficult to define and is usually based upon anatomic criteria. However, limbs with advanced skin changes and ulceration are more likely to have a combination of reflux and obstruction than either abnormality alone.
8The diagnosis of chronic venous disorders has historically relied on a number of clinical tests for venous reflux, such as the Trendelenburg test, supplemented by the findings on continuous wave (CW) Doppler examination.
9,
10 Although sensitive,
both the clinical tests and CW Doppler have limited specificity, leading to selection of inappropriate procedures in a significant number of limbs
9,
11 Not surprisingly, such tests have largely been supplanted by other studies.
Appropriate evaluation of the patient with venous disease depends on the patient’s presentation and anticipated treatment plan. Patients with mild symptoms controlled with compression stockings and for whom no intervention is planned do not require an extensive workup. Similarly, it is generally agreed that tests to exclude truncal reflux in patients with telangiectasias are not needed. In contrast, a more precise assessment of the anatomic distribution of venous reflux and obstruction is required in patients in whom the diagnosis is uncertain or for whom intervention is planned. Evaluating the results of an operative procedure requires a more quantitative physiologic test. Unfortunately, most of the diagnostic tests for chronic venous disease display the fundamental dichotomy of providing either anatomic or hemodynamic information. Imaging-based studies such as descending venography, duplex ultrasonography, and intravascular ultrasound (IVUS) can localize reflux and obstruction to specific venous segments and play an important role in planning interventional procedures. However, such studies provide little information regarding the hemodynamic importance of such pathology or improvement following intervention. Physiologic tests, in contrast, provide a global assessment of venous hemodynamics while providing very little anatomic information. Just as the ankle-brachial index and arteriography play complementary roles in evaluating the lower-extremity arterial system, a combination of physiologic and anatomic studies may be necessary to completely characterize the extent of chronic venous disease in an individual patient.
Descending venography and ambulatory venous pressure have historically been the gold standards for the anatomic localization and hemodynamic quantification of reflux. Although descending venography may still have a role in situations such as venous valve reconstruction, it does have several limitations. It provides little information regarding the great and small saphenous veins, may be limited by the presence of competent proximal valves, and can give false-positive results related to hyperbaric contrast streaming past normal valves. Furthermore, the phlebographic grade of reflux is not precisely related to the severity of disease. In contrast, ambulatory venous pressure measurements are physiologic and, to a certain extent, do correlate with the incidence of ulceration.
12,
13 However, as the test measures only global hemodynamics, it requires reflux at multiple sites, may be insensitive to isolated segmental reflux, and cannot precisely localize reflux to specific venous segments. The measurement of dorsal foot vein pressures also appears to be relatively insensitive to proximal venous obstruction.
14 Furthermore, since these methods are invasive, they are not easily repeatable and are not appropriate screening tests for patients with chronic venous disease. Accordingly, these tests have largely been supplanted by a number of noninvasive studies that alternatively provide either primarily hemodynamic or anatomic information.
NONINVASIVE PHYSIOLOGIC TESTS FOR VENOUS REFLUX
Photoplethysmography (PPG) and air plethysmography (APG) are the most widely available physiologic tests for chronic venous disease. Both use measurements of venous volume to detect reflux. Photoplethysmography is based on transmission of infrared light into the skin and the measurement of back-scattered light by an adjacent photoreceptor. As the patient performs ankle flexion maneuvers in the standing position, the recorded waveform shows a rapid fall from the baseline followed by a gradual return with muscular relaxation. The venous refilling time (VRT) is the time required for return to baseline, and a VRT of ≤20 seconds has often been considered abnormal. Although simple and inexpensive, PPG provides little quantitative information. While a normal VRT excludes significant valvular incompetence and may be useful as a screening test, the specificity of an abnormal result is limited and is poorly correlated with the quantity of reflux and severity of disease.
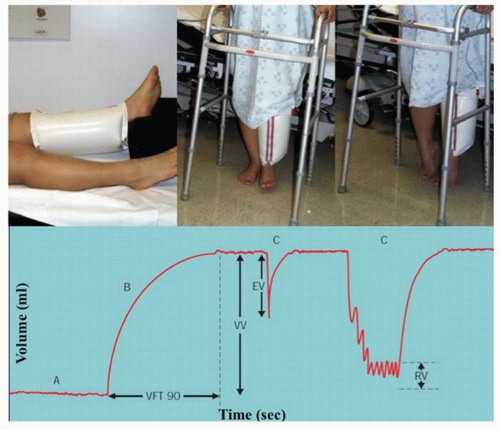
Air plethysmography measures calf volume changes in response to gravity and exercise as a reflection of reflux. Pressures changes with exercise are measured with a calibrated polyvinyl chloride (PVC) air-filled sleeve that surrounds the calf. After obtaining a baseline recording with the patient supine and the leg elevated to 45 degrees, the patient assumes an upright position
(Fig. 21.1). The venous volume (VV) is recorded as the leg fills. The patient is then instructed to plantar flex on both feet to record the ejected volume (EV) followed by 10 tiptoe movements after which the residual volume (RV) is recorded. From this information, it is possible to calculate the venous filling index (VFI = 90% VV/90% venous filling time); the ejection fraction (EF = EV/VV); and the residual volume fraction (RVF = RV/VV). The VFI is an index of global venous reflux, while the EF and RVF reflect calf muscle pump function and ambulatory venous pressure, respectively. The incidence of ulceration increases from 0% with a VFI of less than 5 mL/s to 58% for a VFI greater than 10 mL/s.
15 However, as with other global tests of hemodynamics, APG cannot precisely localize segmental reflux, and the RVF correlates only loosely with the severity of disease. Although the clinical role of APG remains debated, it may have some role in measuring global hemodynamic improvement after venous interventions.
NONINVASIVE VENOUS IMAGING TESTS
Venous duplex ultrasonography has become the most widely used test for the diagnosis of chronic venous disease and indeed has altered its management in many respects. Ultrasound not only permits precise localization of reflux and obstruction, allowing directed venous intervention, but also is used to guide most superficial venous interventions. Both the Society for Vascular Surgery (SVS) and the American Venous Forum (AVF) recommend ultrasound as the first diagnostic test for all patients with suspected chronic venous disease.
16 The SVS/AVF guidelines for venous duplex scanning are shown in
Table 21.2.
In combining B-mode imaging with pulsed Doppler, duplex scanning is capable of characterizing partial and complete anatomic obstruction as well as valvular incompetence in the deep, superficial, and perforating veins. In evaluating reflux, duplex scanning not only provides the anatomic information lacking from global hemodynamic tests but also avoids the venographic limitations of proximal valve competence and hyperbaric contrast, while permitting valve function to be evaluated under conditions that simulate normal calf muscle pump function.
17 It is the most accurate test for identification of isolated but hemodynamically significant reflux in the distal deep venous segments.
18,
19 Although ultrasound is accurate in evaluating chronic infrainguinal venous obstruction, as discussed later in this chapter, it does have some limitations for the diagnosis of iliac vein obstruction.
According to consensus standards, the duplex examination in patients with chronic venous disease should demonstrate the location, competence, and diameters of all saphenous junctions; the extent of saphenous reflux and venous diameters from the groin to the ankle, including the location and
diameter of any incompetent perforating veins; the source of any superficial varices including nonsaphenous sources; anatomic variants including tortuous venous segments and hypoplastic, atretic, or absent veins; and the state of the deep venous system including any reflux or venous obstruction.
20 Formal training using a standardized protocol has been shown to yield the most precise, reproducible results.
21The patient should be examined in a warm environment to avoid venoconstriction that may hinder the detection of reflux. Transducer selection is somewhat dependent on the patient’s body habitus and depth of the veins being imaged, but a linear array transducer with a frequency between 7 and 12 MHz is suitable for most infrainguinal deep and superficial veins. The gain and dynamic gain controls should be adjusted to minimize intraluminal echoes while allowing any thrombus to be visualized. A low flow setting should be utilized, generally with a Doppler velocity range of 5 to 10 cm/s and a low wall filter.
20 The spectral display is adjusted to show retrograde flow above the baseline, and the sweep speed should be slowed to demonstrate the longest duration of reflux.
The deep veins of the lower extremity are first imaged in the reverse Trendelenburg position, using standard ultrasound criteria of compressibility, spontaneous flow, augmentation, and respiratory variation to exclude any component of venous obstruction (see
Chapter 19).
22 The patient then assumes an upright position with the limb relaxed and slightly bent, weight being borne on the extremity contralateral to that being examined. The transverse B-mode image is used to provide anatomic orientation and determine venous diameters. A longitudinal view is usually used to detect reflux, recording the response of the pulsed Doppler spectral waveform to provocative maneuvers, as discussed below, and measuring the duration of reverse flow. Standard sites for the evaluation of reflux, as well as measurement of venous diameters, include the saphenofemoral junction (terminal and subterminal valves); the great saphenous vein at mid-thigh, knee, and mid-calf; and the saphenopopliteal junction and small saphenous vein at mid-calf.
23 The great saphenous vein is clearly recognized by its position in the saphenous fascial compartment, as opposed to the deep veins beneath the muscular fascia and the tributaries in the epifascial compartment
(Fig. 21.2).
23 In evaluating the saphenous veins, attention should be focused on sites of caliber change. Abrupt diameter increases may be associated with entry points of alternative sources of reflux such as the pelvic veins, while decreases are often associated with reflux escape points from the saphenous to the epifascial tributaries.
20 The depth of the saphenous vein from the skin is also an important consideration when endovenous thermal ablation is contemplated (see
Chapters 28 and
29). All varicosities should be traced back to their point of origin. A thorough assessment of deep venous reflux is an integral part of the diagnostic evaluation for chronic venous disorders. If associated with overlying varices, skin changes, or ulceration, the perforators in the mid-thigh and calf should also be evaluated for diameter and the presence of bidirectional or outward flow. A visual diagram of the superficial, deep, and perforating veins, their tributaries, and communications should be provided in addition to the text report
(Fig. 21.3). As they are a source of significant variability, the report should also detail the time of day the study was performed, the position of the patient, and provocative maneuvers used to elicit reflux.
21 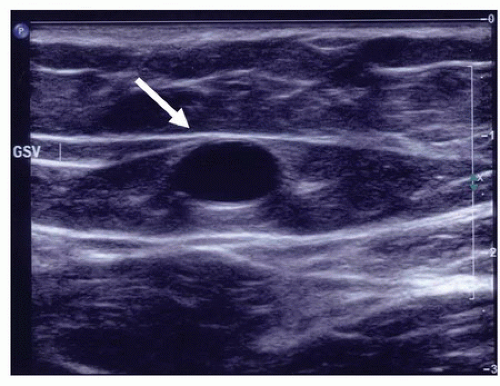


 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access