Fig. 6.1
Chest X-ray showing reduced vascular markings throughout left lung caused by large pulmonary embolism
Transthoracic echocardiography is often the first objective indication as to the presence of elevated pulmonary pressures or right ventricular compromise. Current technology allows for estimates of pulmonary artery systolic pressure (using Doppler analysis of the degree of tricuspid regurgitation), along with cardiac output and RV performance [59]. Enlargement of the right heart chambers, tricuspid regurgitation as a result of this chamber enlargement, flattening or paradoxical motion of the interventricular septum, encroachment of an enlarged right ventricle on the left ventricular cavity, and impaired left ventricular diastolic dysfunction that it is not the result of primary left ventricular or valvular heart disease are findings in patients with significant pulmonary hypertension [60, 61]. Contrast echocardiography using intravenous agitated saline can detect the presence of an intracardiac shunt, such as a patent foramen ovale or a previously undetected septal defect. In a patient with symptomatic chronic thromboembolic disease who has an echocardiogram that shows only minimal elevation of pulmonary artery pressures or modest right ventricular compromise at rest, an exercise stress echocardiogram may demonstrate a substantial rise in pulmonary artery pressures or dilatation of the right ventricle.
To a large extent, computed tomographic (CT) angiography of the pulmonary vessels has replaced ventilation-perfusion (V/Q) scintigraphy in the evaluation of patients with suspected acute pulmonary embolic disease. However, the V/Q scan continues to provide essential information in the pulmonary hypertensive patient, and can often be the first indication that chronic thromboembolic disease should be considered. This study serves as a valuable screening test for this disease [62]. In a single center, retrospective survey comparing V/Q scanning with CT angiography in 227 pulmonary hypertensive patients, there was a sensitivity of 97.4 % for V/Q scanning compared to 51 % for CT angiography in the detection of chronic thromboembolic disease [63]. In a more recent study of 12 CTEPH patients, Soler and colleagues demonstrated that SPECT perfusion scintigraphy was more sensitive in detecting obstructed vascular segments when compared to CT pulmonary angiography, with a sensitivity of 62 ± 4.1 % versus 47.8 ± 2.9 %, respectively [64]. Further, the interpretation of an abnormal perfusion pattern can assist in the differentiation between disorders involving the central or proximal vascular bed from those primarily affecting the peripheral pulmonary circulation. In chronic thromboembolic disease, at least one and more commonly several segmental or larger mismatched perfusion defects are present. For those patients with small vessel pulmonary vascular disease, perfusion scans either are normal or exhibit a “mottled” appearance characterized by nonsegmental defects [65, 66]. Exceptions include cases of pulmonary veno-occlusive disease or pulmonary capillary hemangiomatosis in which multiple, larger mismatched defects have been reported [67, 68]. As important has been the observation that a relatively normal perfusion pattern on V/Q scan excludes the diagnosis of surgically accessible chronic thromboembolic disease (Fig. 6.2).
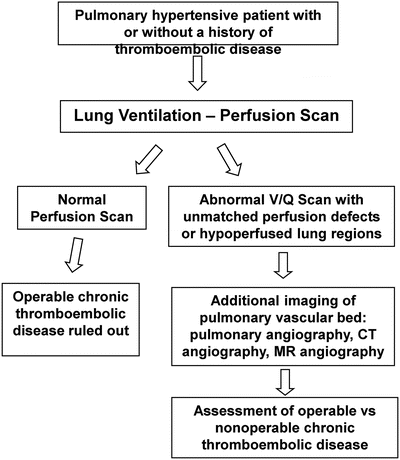

Fig. 6.2
Diagnostic approach to the evaluation of CTEPH. CT computed tomography, MR magnetic resonance. Adapted from Hoeper et al. [62]
It has also been established that the magnitude of perfusion defects exhibited by CTEPH patients with operable disease may understate the degree of pulmonary vascular obstruction determined by angiography [69]. The plausible explanation for this finding is that during the process of thrombus organization, proximal vessel thromboemboli may recanalize or narrow the vessel in such a manner that radiolabeled macroaggregated albumin may traverse the area of partial obstruction, creating gray zones or regions of relative hypoperfusion. Therefore, chronic thromboembolic disease should be considered and further evaluation for operable disease should proceed even if the V/Q scan demonstrates a limited number of mismatched perfusion defects, or lung regions which are relatively “hypoperfused.”
The next step in the evaluation of patients with suspected chronic thromboembolic disease is to more clearly image the pulmonary vascular bed, to not only establish the diagnosis but also define the extent and location of lesions. Prior to the availability of computed tomographic angiography and magnetic resonance imaging of the chest, conventional pulmonary angiography was the principal means of achieving these goals in the evaluation of patients for pulmonary thromboendarterectomy surgery. It can be considered the gold standard in providing a “map” for surgery, against which other modalities are to be measured. Under essentially all circumstances, a properly performed bi-plane angiogram will provide sufficient information on which to base a decision regarding chronic thrombus location, and as a result, surgical accessibility. The angiographic appearance of chronic thromboembolic disease bears little resemblance to that of the well-defined, intraluminal filling defects of acute pulmonary embolism. Instead, the angiographic patterns encountered in chronic thromboembolic disease reflect the complex patterns of organization and recanalization that occur following an acute thromboembolic event. Several angiographic patterns have been described in chronic thromboembolic disease that correlate with material removed at the time of surgery [70]. These include “pouch defects,” pulmonary artery webs or bands, intimal irregularities, abrupt, frequently angular narrowing of the major pulmonary arteries, and complete obstruction of main, lobar, or segmental vessels at their point of origin (Figs. 6.3 and 6.4). In the majority of CTEPH patients, two or more of these angiographic findings are present, typically involving both lungs.
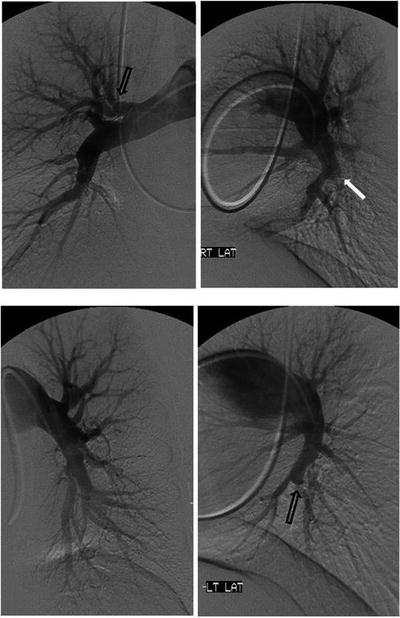
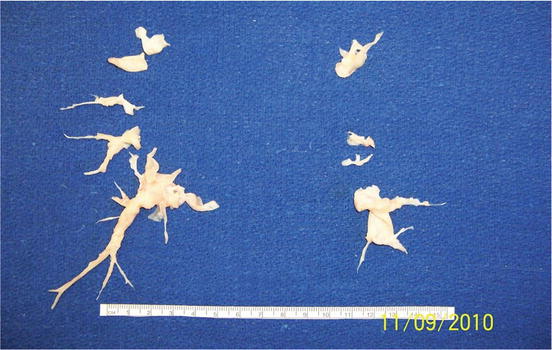

Fig. 6.3
Conventional pulmonary angiogram in a CTEPH patient. Open arrows: “web” narrowing of the proximal segmental arteries. Closed arrow: “pouch” defect occluding the posterior branch to the right lower lobe. Lateral views providing better definition of the pulmonary vascular anatomy that is less apparent on anteroposterior views

Fig. 6.4
Chronic thromboembolic material endarterectomized from the pulmonary vessels depicted in the pulmonary angiogram in Fig. 6.2. Ruler is 15 cm in length
With the advances in computed tomographic (CT) angiography of the chest, and greater availability and use of this technology in assessing the pulmonary vascular bed, CT is playing an expanded role in the evaluation of the pulmonary hypertensive patient for chronic thromboembolic disease. There are a number of CT findings which have been described in patients with chronic thromboembolic disease. These include mosaic perfusion of the lung parenchyma; enlargement of the central pulmonary arteries and right heart chambers; variability in the size of lobar and segmental-level vessels with a reduction in vessel caliber of those involved with chronic thrombi; and peripheral, scar-like lesions in poorly perfused lung regions. With contrast enhancement of the pulmonary vasculature during CT imaging, organized thrombus can be seen to line the pulmonary vessels, often in an eccentric manner. Associated narrowing of pulmonary arteries, web strictures, “pouch defects,” and other irregularities of the intima may also be appreciated [71, 72] (Figs. 6.5 and 6.6). These CT findings are distinct from the intraluminal filling defects of acute thromboemboli and primary pulmonary vascular tumors [73]. With appropriate timing of the intravenous contrast bolus for CTA, opacification of the pulmonary and systemic circulations is possible. In addition to the pulmonary vascular bed, this allows examination of a number of cardiac features including cardiac chamber size, position and shape of the interventricular septum, the presence of congenital cardiac abnormalities, anomalous pulmonary venous drainage, and the size and distribution of collateral vessels arising from the systemic arterial circulation (bronchial arteries off the aorta, coronary vessels) [72, 74]. What remains incompletely validated is the utility of CT angiography in determining operability in certain subgroups of CTEPH patients. This is particularly important as operative techniques allow for resection of chronic thromboembolic material at the segmental vessel level. Clinical experience has demonstrated that the absence of lining thrombus or thickened intima of the central vessels on CT does not exclude the diagnosis of chronic thromboembolic disease or the possibility of surgical intervention. Studies directly comparing CT with pulmonary angiography are limited. In one such study, CT and digital angiography were nearly equivalent in terms of identifying complete vessel occlusion at the segmental level. However, for nonocclusive changes, CT was significantly inferior to angiography [75]. Accuracy of CT has improved with technological advances in scanners. In a recent study of 44 patients with suspected CTEPH, Sugiura and colleagues compared pulmonary digital subtraction angiography with enhanced, electrocardiogram gated 320-slice CT in the detection of thrombi within the pulmonary vascular bed. The sensitivity and specificity of CT to detect chronic thromboembolic lesions were 97.0 % and 97.1 % at the main and lobar vessel level; at the segmental level, the corresponding numbers were 85.8 % and 94.6 %, respectively [76].
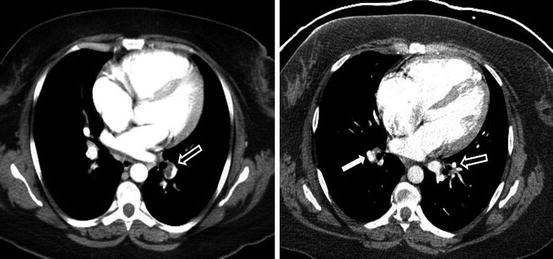
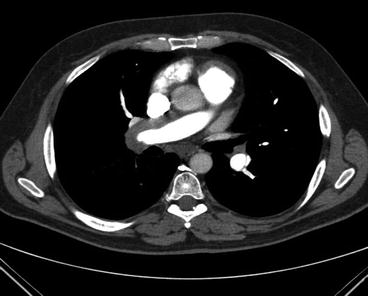

Fig. 6.5
Transition from acute to organized, chronic thromboembolic lesions (open arrows) occurring over 4 months on antithrombotic therapy. Vessel narrowing in the absence of intraluminal defects has occurred. Intimal thickening, a small web and vessel narrowing can be seen at the level of the distal right descending pulmonary artery (closed arrow). The chronic thromboembolic material removed at surgery is pictured in Fig. 6.3

Fig. 6.6
CT angiogram in a CTEPH patient, demonstrating lining thrombus right main and interlobar arteries, with an intravascular web … a remnant of an acute pulmonary embolus … in the left descending pulmonary artery
In addition, there is considerable value for CT in detecting disorders of the pulmonary parenchyma and mediastinum. For CTEPH patients with coexisting interstitial lung disease or emphysema, CT will be able to define the extent and location of the parenchymal lung process. Reperfusion of severely diseased lung parenchyma following an endarterectomy is likely to result in an undesirable outcome, and thereby should exclude a patient from surgical consideration. For those patients whose V/Q scan demonstrates absence or near-complete absence of perfusion to an entire lung, CT is an essential study to rule out extrinsic pulmonary vascular compression from mediastinal adenopathy, fibrosis, or neoplasm [77].
Expanding experience using magnetic resonance (MR) imaging and magnetic resonance angiography (MRA) to visualize the pulmonary vascular bed in patients with chronic thromboembolic pulmonary hypertension has established this imaging modality as a reliable means to determine surgical candidacy for CTEPH patients [78]. Kreitner and colleagues have shown that contrast-enhanced MRA is able to demonstrate the vascular changes typical for CTE disease. In a study of 34 CTEPH patients, wall-adherent thromboembolic material involving the central pulmonary arteries down to the segmental level could be demonstrated; intraluminal webs and bands as well as abnormal vessel tapering and “cutoffs” were also detected. Further they showed that MRA was superior to digital subtraction angiography in determining the proximal location of resectable chronic thromboembolic material [79]. An additional study comparing magnetic resonance techniques with conventional contrast angiography involved 29 patients with either CTEPH or idiopathic pulmonary arterial hypertension (IPAH). Nikolaou and colleagues showed that the combined interpretation of MR perfusion imaging and MR angiography led to a correct diagnosis of IPAH or CTEPH in 26 (90 %) of 29 patients when compared to the reference diagnosis based on V/Q scintigraphy, digital subtraction angiography, or CT angiography. The interpretation of MR angiography alone had a sensitivity of 71 % for wall-adherent thrombi, 50 % for webs and bands, and between 83 and 86 % for detection of complete vessel obstruction and free-floating thrombi when compared to DSA or CT angiography [80]. More recently, Rajaram and colleagues retrospectively evaluated the accuracy of contrast-enhanced MR angiography compared to CT pulmonary angiography in a group of 53 CTEPH patients. The sensitivity and specificity of MRA in detecting proximal and distal vessel chronic thromboembolic disease were 98 and 94 %, the sensitivity of MR diagnosis of central vessel disease considerably improved when images were analyzed with unenhanced proton MRA (50–88 %). Overall, MRA identified more stenosis, poststenotic dilatation, and occlusive lesions when compared with CT angiography in this particular study [81]. However, in a small study of 24 CTEPH patients in whom all three diagnostic modalities were performed within a 3-day period, Ley and colleagues compared digital subtraction angiography, ECG-gated multidetector CT angiography, and contrast-enhanced MR angiography in the detection of vascular changes associated with chronic thromboembolic disease. Based on comparison with reference standards, it was concluded that multidetector CT provided the best image quality and highest level of sensitivity and specificity for detection of vascular abnormalities at the main, lobar, and segmental levels [82].
Additional features of magnetic resonance imaging that can be useful in the evaluation of CTEPH patients include cine imaging which allows an assessment of RV and LV function, providing data on end-systolic and end-diastolic volumes, ejection fraction, and muscle mass [83, 84]. Furthermore, phase contrast imaging may be used to measure cardiac output, along with pulmonary and systemic arterial flow. In CTEPH patients undergoing PEA, this technique has been used to measure changes in aortic and pulmonary arterial blood flow before and after surgery [79].
Surgical Approach to Chronic Thromboembolic Disease
The goals in the evaluation of patients with suspected CTEPH are to first establish the diagnosis, and then to determine whether or not pulmonary thromboendarterectomy is feasible. The appropriate interpretation of the angiographic studies reviewed in the previous section determines the proximal extent, and as a result, the surgical accessibility of the organized thrombi present. Equally essential in the determination of whether any particular CTEPH patient is a surgical candidate, particularly as it relates to an assessment of perioperative risks, is defining the severity of pulmonary hypertension and the degree of cardiac dysfunction at rest with right heart catheterization. Early surgical experience supported the observation that patients with severe pulmonary hypertension (pulmonary vascular resistance >1,000 dyn/s/cm−5) bear a greater perioperative mortality risk. Hartz et al. reported that a preoperative PVR over 1,100 dyn/s/cm−5 was associated with 41 % mortality—compared to less than 6 % if PVR was less than 1,100 dyn/s/cm−5 [85]. Dartevelle and colleagues reported an increased postoperative mortality of 20 % for patients with preoperative PVR over 1,200 dyn/s/cm−5 compared to 4 % mortality if the preoperative PVR was less than 900 dyn/s/cm−5 [86]. Although the San Diego group has reported a declining overall operative mortality risk following thromboendarterectomy surgery over the past several years, the most recent report reveals that in 500 patients operated between 2006 and 2010, those patients with a preoperative PVR > 1,000 dyn/s/cm−5 experienced a mortality rate of 4.1 % compared to 1.6 % in those patients with a PVR less than 1,000 dyn/s/cm−5 [25].
For symptomatic CTEPH patients with minimal pulmonary hypertension at rest, exercise hemodynamic measurements should be undertaken. In these cases it is likely the normal compensatory mechanisms of recruitment and dilation of the pulmonary vasculature have been overcome, and with exercise, an elevation in pulmonary artery pressure as cardiac output increases can be observed. This hemodynamic information provides objective evidence to explain an individual’s symptoms, and may reflect a reduction in pulmonary vascular reserve from chronic thrombotic obstruction of a limited amount of the pulmonary vasculature, or equally plausible, an indication of a clinically relevant stage in the development of severe CTEPH in which there is coexisting small vessel hypertensive changes.
There are a number of considerations in the determination of whether an individual patient with CTEPH might be a candidate for thromboendarterectomy surgery (Table 6.1). Despite advancements in diagnostics and an expanding surgical experience, there remains considerable subjectivity in this assessment. The most critical factor determining operative candidacy is the presence of surgically accessible chronic thromboembolic lesions, as established by angiographic (conventional, CT, MR) studies. However, interpretative experience and knowledge of surgical capabilities at any specialized clinical center will dictate what lesions can be endarterectomized. As surgical experience is gained, not only is it possible to resect main pulmonary artery and lobar level disease, but also more distal, segmental chronic thromboembolic lesions [25]. Although early experience with PTE surgery focused on the treatment of patients with pulmonary hypertension and right heart dysfunction, indications for surgical intervention have expanded to include those patients with chronic thromboembolic disease who experience exertion-related pulmonary hypertension, as well as those who are primarily symptomatic from elevated dead space ventilation [20].
Table 6.1
Selection of patients for pulmonary thromboendarterectomy
• Surgically accessible CTEa disease (main, lobar, segmental) with: |
1. Pulmonary hypertension and right heart dysfunction, or |
2. Exertion associated pulmonary hypertension, or |
3. Elevated dead space ventilation |
• Relative contraindications: |
1. Pulmonary hypertension out of proportion to the extent of CTE present |
2. Endarterectomy of CTE lesions not expected to improve pulmonary hemodynamics |
3. Comorbidities to a degree post-PTE hemodynamic and functional benefits would not be realized |
As important is the recognition when surgical intervention might be ill-advised. Patients exhibiting significant comorbidities (e.g., severe emphysema, end-stage malignancies) that not only place them at extraordinary perioperative risk, but would prevent the hemodynamic and functional status benefit following PTE surgery from being realized should not be considered operative candidates. Furthermore, when the degree of pulmonary hypertension appears out of proportion to the extent of accessible chronic thromboembolic disease apparent by angiography, and surgical resection is not expected to result in a substantive improvement in pulmonary hemodynamics, surgery should be avoided. With appropriate waveform analysis, data obtained with right heart catheterization has the potential to objectively assess the degree of concurrent small vessel disease in CTEPH, essentially “partitioning” the different elements (proximal versus distal) of pulmonary vascular resistance. Though specialized equipment is required and obtaining adequate occlusion waveforms may be difficult in this patient population, the available data from this technique underscores the heterogeneity of pulmonary vascular lesions present in CTEPH. Patients with operable disease usually exhibit a greater degree of upstream resistance (Rup) [87]. This information may further predict outcomes following endarterectomy. In a small series of 26 CTEPH patients, Kim and colleagues, utilizing pulmonary artery occlusion waveform analysis, demonstrated an inverse correlation between the percent upstream resistance and postoperative mean pulmonary artery pressure and pulmonary vascular resistance. In addition, all 4 deaths in this series occurred in patients in whom the Rup was less than 60 % [88]. This finding, however, was not observed in Toshner’s study, where both CTEPH patients who died following endarterectomy exhibited an upstream resistance of 68 and 73 % [87].
Pulmonary thromboendarterectomy is the unique surgical intervention to remove obstructive, adherent chronic thromboembolic lesions from within the pulmonary vascular bed. During the three decades since the first attempts in the early 1960s to surgically treat CTEPH, there were a number of essential technical modifications which have led to the current success of this procedure [89–91]. Performance of a median sternotomy provides access to the central pulmonary vessels of both lungs, while avoiding disruption of the extensive bronchial artery collateral circulation and pleural adhesions that may develop following longstanding pulmonary arterial obstruction. Furthermore, if additional cardiac procedures are necessary, such as coronary artery bypass or valve surgery, there is access to the heart via the sternotomy [92]. The use of cardiopulmonary bypass with periods of hypothermic circulatory arrest is a mainstay of the surgical procedure. Deep hypothermia provides for tissue protection, while intermittent circulatory arrest periods avoids back-bleeding from bronchial artery to pulmonary artery anastomoses and provides the necessary bloodless field to perform the meticulous and optimal dissection of chronic thromboembolic material from the pulmonary vessels. The importance of this phase of the operation has grown as dissection from the segmental arteries has become technically feasible, making adequate visualization of these more distal vessels imperative [25]. Modifications of this technique have been employed with the intent of minimizing neurologic risks of deep hypothermia and circulatory arrest periods. Those described include the use of moderate hypothermia (23–32 °C), aortic bronchial artery occlusion with a balloon catheter, shortening circulatory arrest periods (20 min periods to 7 min) with more frequent reperfusion periods, antegrade cerebral artery perfusion with and without total circulatory arrest, and application of negative pressure in the left ventricle [93–97]. Whether any of these technical modifications result in substantive benefits in postoperative outcomes has not been convincingly established. However, within the experience of a single practice over a prolonged period, utilizing moderate hypothermia and shorter circulatory arrest periods, Morsolini and colleagues demonstrated an improvement in postoperative respiratory function with a reduction in ventilator days and postoperative infections, along with a trend toward fewer neurologic events despite an increase in total circulatory arrest times [97].
Published results have shown that the majority of patients undergoing PTE surgery experience both short- and long-term pulmonary hemodynamic improvement as a result of this operation. A reduction in mean pulmonary artery pressure with augmentation in cardiac outcome is a consistent observation, though results vary between experienced centers [25, 29, 98–101]. In a recent report of the surgical outcomes in a series of 500 patients undergoing PTE between 2006 and 2010, Madani and colleagues showed a reduction in PVR from a preoperative mean value of 719.0 ± 383.2 to 253.4 ± 148.6 dyn/s/cm−5 postoperatively. Mean preoperative pulmonary artery pressure was 45.5 ± 11.6 mmHg, declining to 26.0 ± 8.4 mmHg after surgery. The preoperative cardiac output in this group of patients was 4.3 ± 1.4 L/min, improving to 5.6 ± 1.4 L/min postoperatively [25]. As longer term data have become available, the hemodynamic and resultant functional status improvements are sustained in most patients. A favorable impact on long-term survivorship is also noted [102–106].
Though the definition of residual pulmonary hypertension varies between reporting centers, there are patients who do not achieve normal pulmonary pressures and right heart function following PTE surgery. Occurrence estimates vary between 5 and 35 % of operated patients, though long-term information as to what level of residual pulmonary hypertension negatively impacts functional status and survivorship is lacking [102, 105, 107–109]. Possible explanations for this postoperative outcome include residual chronic thromboembolic disease that could not be surgically resected, or a significant amount of coexisting distal vasculopathy.
Mortality rates reported from centers performing PTE surgery have declined over the years, currently in the range of 2.2–11.4 % [25, 98–101], the lower perioperative mortality figures at centers with more extensive experience. For “low risk” patients based on a preoperative PVR < 1,000 dyn/s/cm−5, postoperative mortality rates have been reported to be as low as 1.3 % [110]. However, the presence of residual pulmonary hypertension post-endarterectomy represented a considerable risk factor for doing poorly in the postoperative period; this same study noting a mortality rate of 30.6 % for those individuals left with a PVR > 500 dyn/s/cm−5 following surgery. However, as has been the experience for CTEPH centers worldwide, the overall decline in mortality rates reflects expanding surgical capabilities, better understanding of the natural history of the disease leading to earlier and more selective surgical referral, improved diagnostic techniques, and better coordinated postoperative care.
Medical Therapy for Chronic Thromboembolic Disease
Over the past couple decades, a number of novel pulmonary arterial hypertension (PAH) specific medical therapies, including prostacyclin analogs, endothelin receptor antagonists (ERA), phosphodiesterase-5 (PDE-5) inhibitors, and most recently, stimulators of soluble guanylate cyclase (sGC), have been developed and proven to be effective in the treatment of various types of small vessel pulmonary arterial disease. With the heterogeneity in pulmonary vascular lesions known to exist in CTEPH [43, 111], and especially given the similarities in small vessel pathology shared with idiopathic PAH, efficacy studies have understandably been pursued in subgroups of patients with chronic thromboembolic disease [26]. Acknowledging that the optimal treatment option for patients with CTEPH is a thromboendarterectomy, the most targeted subgroup where medical therapy has been tested includes those patients deemed to have inoperable CTEPH. Other patient groups where PAH specific medical therapy might prove to be of benefit are in post-thromboendarterectomy patients exhibiting residual pulmonary hypertension and as a “therapeutic bridge” in those patients with operable chronic thromboembolic disease who exhibit severe pulmonary hypertension and right heart dysfunction. Only a limited amount of information is available examining disease modifying therapies for each of these indications.
The majority of data addressing medical therapy for inoperable CTEPH come from trials with the dual endothelin receptor antagonist, bosentan. In a meta-analysis involving 11 studies comprising 269 patients (39 patients with persistent pulmonary hypertension following endarterectomy), treatment with bosentan was associated with an improvement in 6-min walk distance (6MWD) of 35.9 m after 3–6 months of therapy, with further modest gain of 21 m at 1 year for patients receiving drug for a more extended period. Approximately 25 % of patients experienced an improvement in NYHA functional class at 3–6 months. Hemodynamic data available from 7 studies (185 patients) revealed a weighted improvement in cardiac index (0.23 L/min/m2) and weighted reductions in mean pulmonary artery pressure (2.62 mmHg); 5 studies (164 patients) reported a weighted mean reduction of PVR -159.7 dyn/s/cm−5 (20 % of baseline) [112]. The only randomized controlled trial examining the efficacy of bosentan in inoperable CTEPH was reported in 2008. Jais and colleagues enrolled 157 patients (bosentan use in 77 patients), with approximately 28 % having previously undergone PTE surgery. Compared to baseline, 16-week treatment with bosentan resulted in an improvement in pulmonary hemodynamic parameters: a 24.1 % reduction in PVR, a decline in total pulmonary resistance (treatment effect: −193 dyn/s/cm−5) with a rise in cardiac index (treatment effect: 0.3 L/min/m2). There was also a decrease in NT-proBNP levels (−622 ng/L) in the bosentan-treated patients relative to those receiving placebo. However, at 16 weeks, there was no definable improvement in exercise capacity (6MWD) and no statistically significant treatment effect of bosentan on WHO functional class [113].
Available studies examining the efficacy of other classes of PAH specific medical therapy for patients with inoperable CTEPH are even more limited. In a double-blind, placebo-controlled, 12-week pilot study, Suntharalingham and colleagues enrolled 19 patients with inoperable CTEPH, assessing the benefit of sildenafil (9 patients receiving drug) in this group. Although there was no significant difference detected in 6MWD (primary end point), an improvement in WHO functional class and PVR was noted. Control subjects were then transferred to open-label sildenafil use and reassessed at 12 months. Significant improvement in 6-min walk distance, activity and symptom scores (CAMPHOR), cardiac index, PVR, and NT-proBNP values (1,000 to 811 pg/mL) was noted [114]. In a larger patient group, Reichenberger and colleagues conducted an open-label study of sildenafil (50 mg three times a day) in 104 patients with inoperable CTEPH. After 3 months of therapy, there was a modest decrease in pulmonary vascular resistance (863 ± 38 to 759 ± 62 dyn/s/cm−5), with an increase in 6MWD from 310 ± 11 to 361 ± 15 m; this distance further improved to 366 ± 18 m after 12 months of sildenafil [115]. In a single center uncontrolled observational study, 28 patients with severe inoperable CTEPH were treated with subcutaneous treprostinil. Right heart catheterization was repeated in 19 patients after 19 ± 6.3 months of treatment. Treprostinil therapy was associated with not only a significant reduction in PVR but also an improvement in 6MWD, WHO functional class, BNP levels, and cardiac output. Five-year survival rate was 53 % compared with 16 % in untreated historical controls [116]. The use of intravenous epoprostenol in patients with inoperable CTEPH has also been examined. Cabrol and colleagues retrospectively analyzed 27 patients with inoperable CTEPH who were treated with epoprostenol. After 3 months of therapy, there was a decrease in mean pulmonary artery pressure (56 ± 9 to 51 ± 8 mmHg), total pulmonary resistance (29.3 ± 7.0 to 23.0 ± 5.0 U/m2), and an increase in 6MWD of 66 m. NYHA functional class improved by one class in 11 of 23 patients [117]. Riociguat, a soluble guanylate cyclase stimulator and the first such agent in this unique class of PAH modifying therapies, has been trialed in patients with pulmonary hypertension and inoperable CTEPH. In a multicenter, open-label, uncontrolled phase II trial, 41 patients with inoperable CTEPH (four patients also receiving bosentan) were treated with riociguat for 12 weeks. An overall improvement in 6MWD of 55 m (17.0–105.0) from a baseline of 390.0 m (330.0–441.0) was noted. In a subgroup of 30 patients undergoing follow-up right heart catheterization, there was a significant decline in mean PA pressure (median improvement of -4.5 mmHg from a baseline of 42.5 mmHg), accompanied by a significant decrease in PVR and SVR, along with a rise in cardiac index [118]. A double-blind, placebo-controlled study of 261 patients examining the efficacy of riociguat in the treatment of inoperable, residual, or recurrent CTEPH demonstrated a placebo corrected difference in 6-min walk distance of 46 m at 16 weeks [119]. Positive secondary endpoints included a 246 dyn/s/cm−5 placebo corrected change in pulmonary vascular resistance, and improvement in functional class and NT-proBNP levels. This important study has resulted in specific regulatory approval of riociguat to treat inoperable or residual thromboembolic pulmonary hypertension.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


