Chapter 7
Chronic Lower Limb Ischaemia
Harjeet Rayt and Robert S.M. Davies
Department of Vascular and Endovascular Surgery, Leicester Royal Infirmary, UK
Introduction
The phrase ‘chronic lower limb ischaemia’ refers to symptoms and signs of leg ischaemia that have been present for at least 2 weeks. This covers a wide range of disease from asymptomatic peripheral vascular disease (PVD) and intermittent claudication (IC) at one end of the spectrum to critical limb ischaemia (CLI) at the other (Table 7.1). For the purposes of this chapter, these extremes of disease will be considered individually. While the prevalence of symptomatic PVD in people aged 55–74 years is approximately 5%, 8% and 20% of this age group have asymptomatic major and minor disease, respectively. An important point to remember when assessing patients with chronic leg ischaemia is that the affected leg is part of a patient who must be assessed as a whole, both medically and socially.
Table 7.1 Fontaine classification of severity of peripheral arterial disease.
| Stage | Symptoms | Description |
| I | Asymptomatic | PVD present but no symptoms |
| II | Intermittent claudication | Cramp-like pain in leg muscle on activity, eased with rest |
| III | Rest pain | Constant pain in feet (worse at night) |
| IV | Tissue loss | Ischaemic ulceration or gangrene |
Definitions
Peripheral Arterial Disease (PAD)/Peripheral Vascular Disease (PVD). This is a vascular disease caused primarily by atherosclerosis and thromboembolic pathophysiological processes that alters the normal structure and function of the aorta, its visceral branches and the arteries of the lower extremity.
Intermittent Claudication. This is a clinical diagnosis given to the symptoms of painful muscle cramps, usually in the legs, that reliably occurs during exercise and is relieved by rest.
Critical Limb Ischaemia. This is defined as an history of more than 2 weeks of rest pain, ulcers or gangrene attributed to arterial steno-occlusive disease, with an ankle pressure of <50 mmHg or toe pressure <30 mmHg.
Risk factors for chronic ischaemia
The underlying pathophysiological process will predominately be atherosclerosis. Prevalence of arterial disease in the legs will be suggestive of disease in other arterial segments, in particular, coronary (40–60%) and cerebral (25–50%). The risk factors associated with PVD mirror those for ischaemic heart disease. Arterial disease is commonly seen after the age of 55, but in combination with other risk factors, it is increasingly seen at a younger age. Non-modifiable factors include age, race and gender. Modifiable risk factors include smoking, diabetes mellitus, hyperlipidaemia and hypertension.
Assessment
A large proportion of patients with PVD will remain undiagnosed due to either absent or atypical symptoms. The absence of symptoms may be due to the limitation of exercise from other causes (e.g. respiratory disease), mild disease and peripheral neuropathy. Of the symptomatic patients, one-third will present with IC. This is classically a debilitating muscular pain in the calf, thigh or buttock on exertion that occurs predictably at a fixed distance and is relieved by rest within 5 min of stopping. The muscular pain results from anaerobic muscle metabolism from an inadequate arterial supply. Atypical symptoms are common and are described as leg aches, tiredness or weakness; the patient may be able to walk through the pain, and the pain may not completely resolve with rest.
As the disease progresses, the claudication distance (distance at which the patient develops symptoms) declines, and the perfusion to the foot may become sufficiently compromised that the patient complains of ‘rest pain’. This is pain in the foot in the absence of exercise and often initially occurs at night when the affected leg is in an elevated position and tissue perfusion pressure is lowered. The patient often describes being awoken by severe pain finding relief only upon placing the affected foot in a dependent position such that gravity aids tissue perfusion; some patients may only be pain free sleeping in a chair. If this persists for 2 weeks, the patient is said to have developed CLI, which can be confirmed by the aforementioned criteria.
Examination of the patient should address three important questions:
- Is PAD present?
- How severe is the disease and is the foot imminently threatened (CLI)?
- What is the anatomical location of disease, e.g. aorta, femoral artery, multiple arterial segments?
The examination of any patient with suspected chronic lower limb ischaemia should comprise a complete cardiovascular assessment including blood pressure (BP) and pulse assessment in both arms, auscultation of the heart and carotid arteries and palpation of the abdomen for the presence of an abdominal aortic aneurysm.
The lower limbs should be carefully inspected; a common mistake is failure to examine in-between the toes or the heels for occult ulceration/tissue loss. In contrast to the acutely ischaemic limb, skin temperature may be an unreliable sign in CLI, but a unilaterally cold leg should alert the physician to the possibility of PAD. Capillary refill time and Buerger’s test are also useful indicators of disease. A critically ischaemic foot may appear red and ‘healthy’ particularly in patients who have had their foot in dependence awaiting the consultation (Figure 7.1). This is known as a ‘sunset’ foot and occurs due to maximal skin capillary dilatation and is often mistaken for cellulitis by the unwary. However, on elevation of the foot, the severity of the ischaemia is revealed through skin pallor and venous guttering (Figure 7.2). Progression of the disease may lead to a blue/purple discolouration (Figure 7.3) before fixed changes such as gangrene become apparent (Figure 7.4).
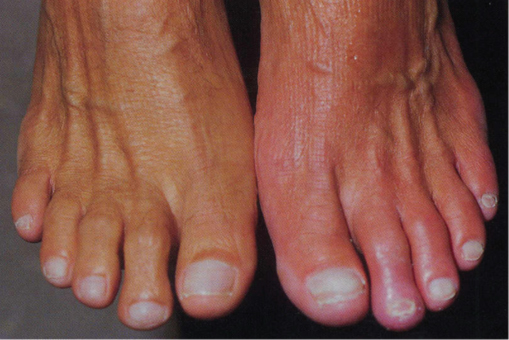
Figure 7.1 Photographic demonstration of a critically ischaemic ‘sunset’ foot (patient’s left foot).
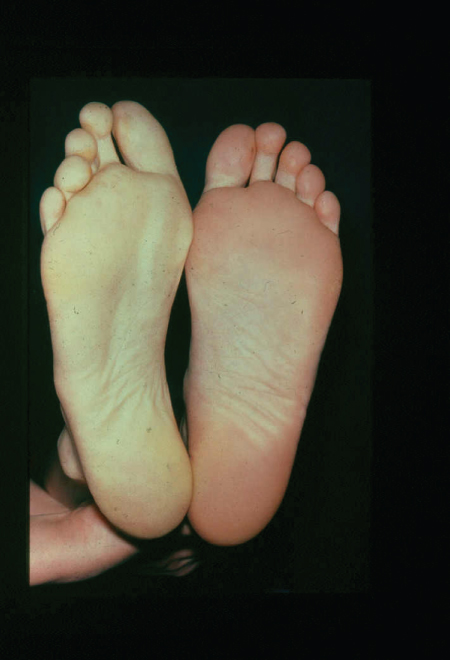
Figure 7.2 Photographic demonstration of elevation-induced pallor of the right leg indicating peripheral vascular disease. This is the basis of Buerger’s test.
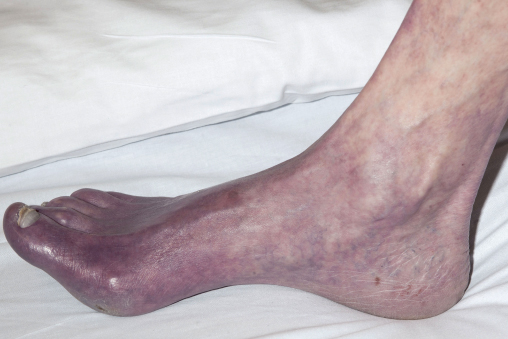
Figure 7.3 Photographic demonstration of a critically ischaemic foot with impending gangrene.
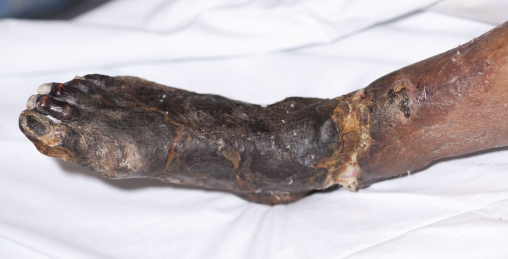
Figure 7.4 Photographic demonstration of dry gangrene.
Pulses throughout the lower limb should be assessed and documented. The muscle group affected by claudication is typically one anatomical level below the causative arterial stenosis/occlusion, i.e. the absence of palpable arterial pulses distal to the common femoral arterial denotes ‘femoro-popliteal’ arterial disease and may present as calf claudication, whereas weakness/absence of all lower limb arterial pulses including the common femoral pulse indicates aorto-iliac disease and often presents with calf, thigh and sometimes buttock symptomatology. It is important to remember that pedal pulses may be present at rest in patients with an isolated iliac system stenosis, but during exercise, the pulses may disappear as peripheral vasodilatation occurs.
Investigations
Hand-held Doppler examination gives vital information on the presence or absence of the peripheral pulses and should complete any vascular examination. Quantitative assessment can be made by performing an ankle brachial pressure index (ABPI) with a normal ratio being 0.9–1.3; ABPI values of 0.4–0.9 signify mild to moderate PAD, and values less than 0.4 are associated with severe PAD. Falsely elevated values (>1.3) may occur in patients with ‘calcified’ vessels (diabetics, chronic renal disease) and this should alert the clinician to the presence of PAD, although not necessarily flow-limiting PAD. The ABPI is usually performed at rest in the supine position. A simple advancement on baseline ABPI is to perform an ABPI after exercising to the onset of pain; the ‘walk test’. A drop in the ABPI of ≥20% after exercise indicates the presence of flow-limiting PAD.
If vascular disease is suspected, the arterial tree can be imaged using duplex ultrasound scan (DUSS), or more complex investigations such as computed tomography arteriography (CTA), magnetic resonance arteriography (MRA) or percutaneous angiography (which can also be used as a therapeutic tool). DUSS is inexpensive, readily available and non-invasive, although it remains operator dependant. CTA is useful for assessing supra-inguinal arteries (aorto-iliac) that may be poorly visualised with DUSS due to vessel tortuosity, patient adiposity or overlying bowel gas. CTA is also readily available and can be used to create 3D reconstructions of the arterial tree, which aid in management planning. A major limitation in the use of CTA is the requirement for contrast media, which poses a risk of acute kidney injury (AKI) and allergic reaction. MRA also provides excellent imaging of the peripheral arterial tree with or without contrast (gadolinium). However, it is less readily available, contraindicated in patients with certain types of metallic implants (pacemakers, stents) and not tolerated by claustrophobic patients. Digital subtraction angiography (percutaneous angiography) (DSA) is considered the ‘gold standard’ as it provides dynamic arterial flow information for the treating physician and can be combined with a definitive intervention in the form of angioplasty when indicated. However, it is invasive, expensive and associated with complications in ≤5% of patients that include AKI (from contrast use), allergic reaction, false aneurysm, arterial dissection, arteriovenous fistulation and embolisation.
Treatment
The overall aim of treating PVD is to (i) reduce the patient’s cardiovascular risk through the modification/elimination of atherosclerotic risk factors and (ii) improve/relieve the patient’s presenting symptomatology.
Risk factor modification
PVD is a strong marker of cardiovascular risk with over half of the patients having coronary artery disease; the extent of coronary artery disease has been shown to independently correlate with the patient’s ABPI. Furthermore, more than one-quarter of patients with evidence of lower limb PVD have corresponding level of atherosclerotic disease affecting their carotid and renal arterial territories. Thus, it is not surprising that patients with PVD have a 6-fold increase in mortality from cardiovascular disease than their disease-free counterparts; overall major cardiovascular event rate = 5–7% per year (Figure 7.5).
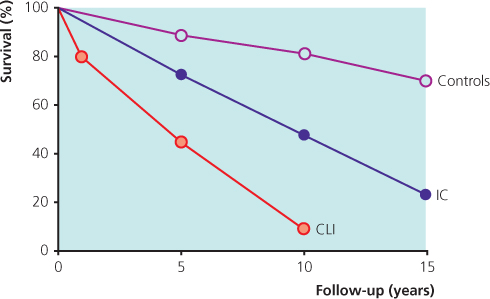
Figure 7.5 Survival in patients with IC and CLI compared with age-matched healthy controls. Source: Norgren et al. (2007). Reproduced by permission of Elsevier.
Cardiovascular risk modification involves smoking cessation, diagnosis and control of diabetes mellitus and hypertension, diet and weight management, lipid modification and anti-platelet therapy. Smoking cessation can be achieved through a number of methods using formal cessation programmes and nicotine replacement therapy. The European Society of Cardiology recommends the maintenance of BP at ≤140/90 mmHg (with a systolic of ≤130 mmHg in diabetics and those with chronic kidney disease). Treatment with angiotensin-converting enzyme inhibitors (ACEi) is recommended as first line therapy due to benefits seen beyond simple BP control. Importantly, beta-blockers are not contraindicated, and a recent meta-analysis found them not to affect claudication distance. HMG-CoA reductase inhibitors (statins) have been shown to reduce major cardiovascular events independent of age, gender or serum lipid levels. Anti-platelet agents have also shown a reduction in incidence of vascular mortality, and non-fatal MI/CVA of up to one-fifth.
‘All patients with PVD should be commenced on anti-platelet therapy and a statin unless contraindicated and have appropriate measures instigated to control hypertension and diabetes’.
Symptom relief
Intermittent claudication
Asymptomatic disease does not require any treatment other than cardiovascular risk modification. Patients with IC should undergo a period of conservative management with the aim being symptom improvement by increasing claudication distance and reducing pain. This can be achieved through two main routes: exercise therapy and pharmacotherapy. Exercise not only improves symptoms but also works on multiple levels to reduce cardiovascular risk. NICE advises a formal programme that consists of 2 h of exercise a week for 3 months. The mechanism of action is unknown but theories regarding metabolic adaptation of muscle, change of muscle morphology and action, increased capillary blood flow and collateralisation have all been postulated. A number of studies have demonstrated that a structured exercise programme can lead to a sustained significant increase in peak walking distance without the need for invasive intervention.
Historically, a large number of drugs have been used to treat IC, but the evidence behind their use has been poor, and any benefits seen were minimal and short-lived. Currently, NICE recommends the use of naftidrofuryl oxalate only when exercise therapy has failed and the patient does not want invasive treatment. In the authors’ experience, little ‘real-life’ benefit is gained through the use of pharmacotherapy for IC (Figure 7.6).
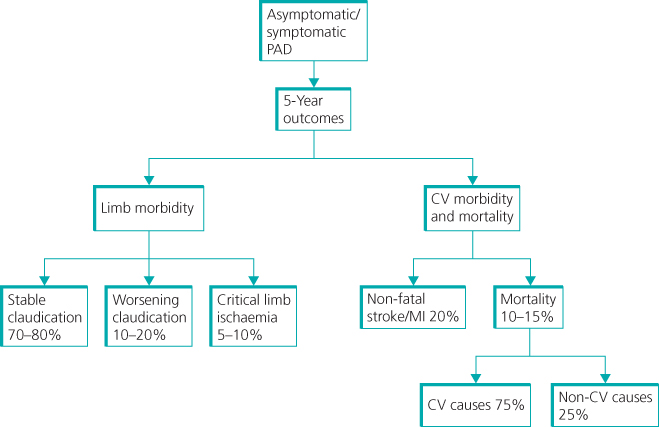
Figure 7.6 Intermittent claudication prognosis for patients and their legs.
A number of patients will return to their treating doctor complaining that there has been little or no improvement in their presenting symptomatology. In these cases, particularly in those patients who are suffering from severe lifestyle-limiting claudication, invasive intervention in the form of angioplasty or bypass surgery may be considered. However, this must be discussed at length with the patient as resulting complications may lead to limb loss.
Critical limb ischaemia
Long-term survival of patients presenting with CLI is generally poor due to their advanced co-existent cardiovascular and cerebrovascular arterial disease. Twenty percent will die within a year and >50% die within 5 years. The aim of the treatment is ‘life and limb’ salvage (Figure 7.7).
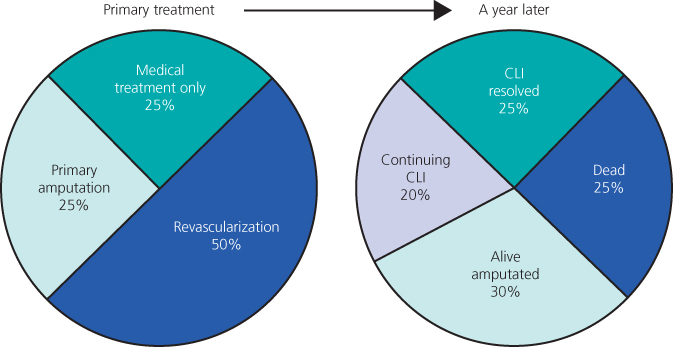
Figure 7.7 Pie chart illustrating the fate of patients presenting with critical limb ischaemia. Source: Norgren et al. (2007). Reproduced by permission of Elsevier.
Assessment in a vascular unit with a multidisciplinary approach is essential. Risk factor modification, wound care, footwear and rehabilitation can all be discussed before and after revascularisation. The mainstays of revascularisation are angioplasty and arterial bypass surgery. Choice of treatment depends on patient co-morbidities, pattern of disease, availability of a vein conduit and patient preference. Angioplasty is usually possible for iliac artery lesions up to 10 cm and superficial femoral artery (SFA) disease up to 15 cm (Figure 7.8). Longer lesions and crural disease can be attempted but require a greater level of skill. Bypass is generally performed using autologous long saphenous, cephalic or basilic vein grafts. In general, endovascular intervention is the preferred option for patients with significant co-morbidities, a life expectancy <2 years, grossly ulcerated/infected limbs rendering the integumentum unsuitable for surgical incisions and/or a lack of a suitable autologous vein conduit. Arterial bypass surgery is the preferred option in patients expected to survive beyond 2 years and is associated with 5-year limb salvage rates approaching 80% (Figure 7.9). In the authors’ practice, a ‘hybrid’ approach is increasingly being employed where endovascular and open surgical revascularisation techniques are performed concomitantly.
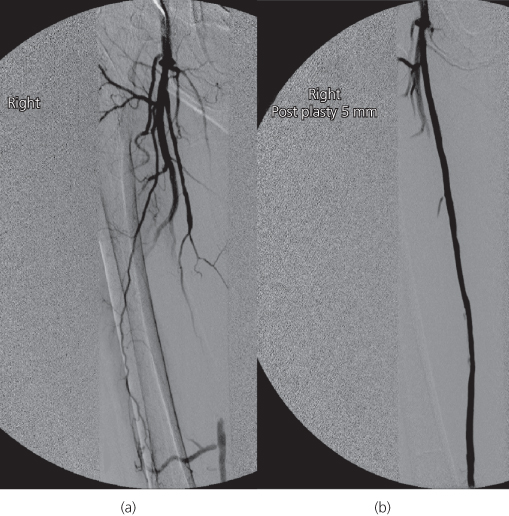
Figure 7.8 Digital subtraction angiogram showing before (a) and after (b) images of a long superficial femoral artery (SFA) angioplasty. Note the distal SFA on the left image supplied by collaterals.
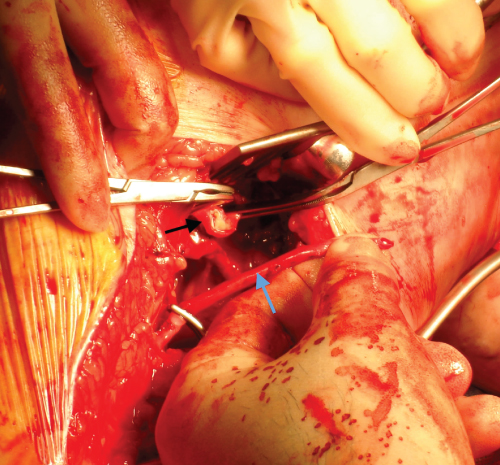
Figure 7.9 Intra-operative photograph before distal anastomosis of a femoropopliteal bypass graft. The blue arrow identifies the long saphenous vein conduit. The black arrow identifies the popliteal artery that has been opened with a longitudinal arteriotomy.
Primary amputation may be considered in patients with extensive tissue loss, flexion contractures of the knee and hip and severe life-threatening sepsis. These decisions are best made involving the patient, relatives and the multidisciplinary team.
Occasionally, the option of revascularisation is not possible and the patient declines a major amputation. These patients are best placed on a formal palliative/end of life care pathway. NICE recommends using the analgesic ladder and recruiting the help of pain specialists; the majority of patients will require opioid analgesia for symptomatic relief. Chemical sympathectomy is not recommended by NICE.
Novel therapies
Spinal cord stimulation is a technique used for the relief of chronic pain. An epidural electrode is inserted and—using the pain gate theory—stimulation of the spinal cord at L3/4 has been shown to reduce pain and produce a sensation of warmth and paraesthesia in the limb. Results of this therapy are equivocal, and because of its high cost and side effects, it should only be offered by the pain team. Intermittent pneumatic compression has been shown to alleviate pain and healing by increasing blood flow, although the mechanism is not fully understood. While this remains a relatively cheap option, its effectiveness is yet to be proven. Iloprost, a synthetic analogue of prostacyclin PGI2, has also been used to improve distal blood flow by causing a vasodilatory effect. However, it is usually administered intravenously, requiring hospital admission and can cause profound hypotension and headaches. Although studies have suggested a reduction in death and amputation with its use, these are not long-term effects. Angiogenesis and gene therapy are also being considered but these techniques at present remain research interests.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


