Stage
GFR (ml/min/1.73 m2)
HT
Kidney damage
CKD
CVD risk
Glomerular filtration rates
1
≥90
+
0
0
±
≥90
±
+
+
+
2
60–89
0
0
±
±
60–89
±
+
+
+
3
30–59
±
+
+
+
4
15–29
+
+
+
+
5
<15
+
+
+
+
Presence of kidney damage classified as CKD irrespective of GFR level
GFR ≥90 ml/min/1.73 m2
Individuals with GFR ≥90 ml/min/1.73 m2 may or may not have CKD depending on the presence of evidence of kidney damage. Those with this level of renal function with or without hypertension and no markers of kidney damage are considered to have no CKD, although an elevation of BP may predispose to adverse renal and cardiovascular outcomes [9]. However, GFR ≥90 ml/min/1.73 m2 with markers of kidney damage are considered to have CKD and are classified as stage 1.
GFR = 60–89 ml/min/1.73 m2
Individuals with GFR = 60–89 ml/min/1.73 m2 are classified as stage 2 and may or may not have CKD. Such levels of GFR are frequently observed at extremes of age (infants and elderly). Whether such GFR levels without markers of kidney damage suggest CKD is unclear. However there is no definite evidence of increased risk of progression to CKD and or cardiovascular events in such individuals [9].
GFR <60 ml/min/1.73 m2
All individuals with a GFR <60 ml/min/1.73 m2 for over 3 months are classified as having CKD independent of the presence of kidney damage [9]. These individuals, who have lost over 50 % of their renal function, appear to be at increased risk of cardiovascular complications [9].
21.2.2.2 Kidney Damage
21.2.2.3 Renovascular Associations
The advent of adequate renal replacement therapy (dialysis and renal transplantation) over the past several decades has been associated with increased life span of patients with end-stage renal disease (ESRD). As those patients survive longer, they tend to develop and die of cardiovascular disease [11, 12].
Compared to the general population, the mortality rate from CVD is 500-fold higher in younger patients on dialysis and five times greater among older patients [11, 12].
The increased risk of poor cardiovascular outcome is not limited to patients with ESRD. Patients with early CKD are more likely to die from cardiovascular comorbidities than to progress to ESRD [13, 14]. Similarly, patients with reduced GFR tend to die from cardiovascular disease than to develop ESRD [13, 14].
21.3 Spectrum of Vascular Diseases in Adults
21.3.1 Renovascular Associations
The pathophysiologic mechanisms of the exclusively elevated cardiovascular morbidity and mortality in CKD/ESRD have not been completely elucidated. Patients with CKD have a high burden of cardiovascular risk factors [15, 16]. They share both traditional and uremia-related risk factors [15, 16]. As a result, CKD populations display a wide spectrum of vascular pathologies [12, 17]. The kidney appears to act as a perceiver and modulator of cardiovascular disease [18].
Among various system/organ disorders, renal functional impairment is unique in that it involves almost every segment of the cardiovascular system. Vascular changes involve both the macrocirculation and microcirculation [12, 17] (Fig. 21.1).
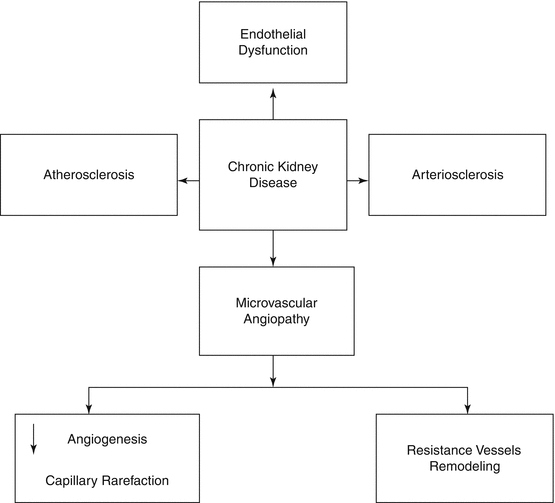

Fig. 21.1
Spectrum of vascular disease in chronic kidney disease
21.3.2 Macrovascular Diseases
21.3.2.1 Atherosclerosis
Atherosclerosis which refers to an intimal disease, more frequent in CKD/ESRD than in nonrenal populations, is characterized by the presence of plaques and occlusive vascular lesions [12, 18, 19]. Calcifications, when present, involve the intima [18, 19]. However, in CKD, the atherosclerotic lesions have a distinct morphology. They are frequently calcified with a relatively increased media thickness, whereas in the general population, they are fibroatheromatous with thickening of the intima [18, 19].
Intimal calcification is observed in older patients with a clinical history of atherosclerotic complications and even before the initiation of dialysis therapy [20]. In contrast, medial calcification occurs more frequently in young and middle-aged patients with CKD, and its severity increases with the degree of uremia-related cardiovascular risk profile [20, 21].
In CKD, as in the general population, traditional cardiovascular risk factors have been postulated to initiate the atherosclerotic process [5, 11]. Among these factors, dyslipidemia appears to be a major determinant [22–24]. However the atherogenic dyslipidemia pattern, which is characterized by increased triglycerides, low high-density lipoprotein cholesterol (HDL-C), and normal or near-normal total serum cholesterol levels, plays a critical role in the pathogenesis of the atherosclerotic process only in patients with mild renal functional impairment [25, 26]. In contrast, in patients with more severe renal functional impairment, the atherogenic dyslipidemia pattern becomes a weaker predictor of atherosclerosis and cardiovascular disease [25, 26]. In moderate to severe renal insufficiency, there appears to be no relationship between renal function and progression of the atherosclerotic process [27]. No difference in atheroma plaque volume and growth could be demonstrated in patients with GFR >60 ml/min/1.73 m2 versus those with GFR <60 ml/min/1.73 m2. These observations suggest that in more severe renal functional impairment, pathobiologic processes other than or in addition to the known traditional factors may be involved in the initiation and progression of cardiovascular disease [26–28].
Atherosclerotic lesions tend to be patchy in distribution along the length of the artery and more frequent in the CKD population and tend to be more occlusive [25–28]. Clinical presentations include ischemic heart disease (angina, myocardial infarction, and sudden cardiac death), cerebrovascular disorders, and heart failure [25, 26].
21.3.2.2 Arteriosclerosis
Arteriosclerosis is a frequent vascular lesion in CKD/ESRD. It is defined as hardening or stiffening of the arteries, particularly the elastic arteries (aorta/major branches and common carotid artery), and is characterized by increased luminal diameter, media thickness, and extracellular matrix, destruction of the elastic lamellae, and extensive medial calcification [20, 21, 29, 30]. These structural modifications, often referred to as remodeling, reduce elasticity and compliance and increase stiffness of the elastic arteries, impairing the cushion function and capacity to smooth out the pulsatile flow associated with intermittent ventricular ejection [29, 30].
Arterial aging impaired renal function and elevated BP levels are the major determinants of increased arterial stiffness [29].
Factors that link arterial stiffness to renal function have not been completely elucidated. Disturbed mineral metabolism associated with renal functional impairment has been postulated to account for remodeling process [20, 28]. In CKD/ESRD, the mineral metabolism, characterized by hyperphosphatemia, increased calcium phosphorous product, hyperparathyroidism, and reduced 1,25 vitamin D(OH) 25, appears to induce medial calcification and arterial stiffening [20, 28, 31–33]. However the relationship between increased arterial stiffness and renal function is not limited to patients with substantially impaired renal function and ESRD. An inverse relationship between arterial stiffness and renal function has been reported in a group of never-treated individuals with mild renal insufficiency (serum creatinine ≥1.47 mg/dl) and normal or mildly elevated BP levels [34]. It has been postulated that in such individuals changes in serum phosphate levels, although still within the normal reference range, may trigger arterial calcification and cause increased arterial stiffness [34].
Ejection of left ventricular stroke volume into stiff elastic arteries results in an increase in amplitude of the systolic and a decrease in diastolic blood pressures, enhanced pulse wave velocity and early return of the reflected pulse wave into late systole, and a wide pulse pressure [35, 36]. Clinically, these alterations in arterial function are associated with isolated systolic hypertension, left ventricular hypertrophy, coronary hypoperfusion, and damage to highly perfused target organs such as the brain and the kidneys. These patients are prone to develop coronary events, heart failure, impaired cognitive function and dementia, and renal dysfunction [35, 36].
21.3.2.3 Vascular Diseases in ESRD/Uremia
Patients on renal replacement therapy often exhibit both atherosclerosis and arteriosclerosis. Both lesions are more severe and more widespread. A significantly higher prevalence of calcified plaques has been reported in the common carotid artery [23, 24, 28, 29, 37].
The arteriosclerotic changes which involve the large central elastic arteries are characterized by luminal dilatation, increased intima-media thickness, and significant increase in arterial stiffness. In uremic patients, the altered arterial stiffness appears to be more apparent in younger than in older individuals, since aging may mask the uremia-related elevation in the wall properties of the vessel [7, 28, 29].
21.3.2.4 Calcific Uremic Arteriolopathy/Calciphylaxis
Calcific uremic arteriolopathy (CUA), also known as calciphylaxis, is a rare life-threatening microvascular calcific disorder which affects predominantly adult patients with ESRD and uremia or with CKD [39, 40]. It is characterized both by specific histopathologic findings and clinical presentation. Histopathologic findings include media calcification of the small- and medium-sized vessels, extravascular calcification, intimal proliferation, microthrombus formation, epidermal ulceration, and subdermal and dermal necrosis [39]. The obliterative vasculopathy also involves both venules and capillaries [39]. The clinical manifestations are characterized by painful, violaceous, mottled skin lesions which may progress to tissue necrosis, nonhealing ulcers, and gangrene, possibly leading to amputation, sepsis, and/or death [39]. Multiple risk factors, such as female gender, diabetes, obesity, warfarin use, hyperphosphatemia, hyperparathyroidism, use of calcium-containing phosphate binders, and severe CKD, have been postulated to act as pathophysiologic factors [39–41]. The prognosis of CUA is poor, being determined by the underlying cardiovascular disease [39, 40].
21.3.2.5 Uremic Cardiomyopathy
Cardiovascular disease (CVD), the leading cause of mortality in patients with end-stage renal disease (ESRD), accounts for over 40 % of all deaths [42]. Of all of these various complications, heart failure, often referred to as uremic cardiomyopathy, appears to be the most frequent [43–46].
The pathophysiologic mechanism of uremic cardiomyopathy has not been completely elucidated. In contrast to the general population, the contribution of atherosclerotic coronary artery disease is less important in CKD [43–46]. In an autopsy study of 94 uremic patients dying from heart failure, presence of coronary heart disease was absent to minimal in 40 % [42].
Hemodynamic, structural, and uremia-related humoral and metabolic factors have been postulated to account for uremic cardiomyopathy. With worsening renal function and onset of heart failure, uremic patients often exhibit hypertension, anemia, and overactive circulation associated with the creation of an arteriovenous fistula in those on hemodialysis treatment, increased arterial stiffening, left ventricular hypertrophy, and dilatation resulting from pressure and volume overload [45, 46]. Structural changes in autopsied uremic hearts include intracardiac coronary artery thickening, decreased myocardial capillary density, increased myocardial fibrosis, and inhibition of apoptosis in hearts of experimental uremic animals [42–46]. Clinically these patients present heart failure resistant to therapy, evidence of myocardial ischemia and arrhythmias, and sudden cardiac death [45].
21.3.3 Microvascular Diseases
21.3.3.1 Impairment of Endothelial Function
Endothelial dysfunction, a major determinant of cardiovascular disease, is frequently reported in CKD/ESRD [47].
Endothelial function is assessed by dilatation of the brachial artery in response to hyperemia or administration of sublingual glyceryl trinitrate (nitroglycerine) [48]. Maximal brachial artery dilatation to hyperemia assesses endothelium-dependent vasodilatation while the response to nitroglycerine evaluates the endothelium-independent vascular function [48].
In CKD, the endothelium-dependent vasodilatation is defective, while the endothelium-independent vascular function remains intact [48].
In CKD, impairment of endothelial function has been reported even in mild decrease in renal function and becomes progressively severe with further deterioration in renal function [48].
Several factors have been implicated in the pathophysiology of endothelial function in CKD [47–50]: (1) impaired nitric oxide release or bioavailability due to accumulation and elevation of asymmetric dimethylarginine (ADMA) levels, (2) oxidative stress associated with accumulation of oxidative stress markers, (3) activation of renin-angiotensin system which induces oxidative stress, (4) chronic inflammation, (5) homocysteinemia, (6) hyperuricemia, (7) dyslipidemia, and (8) deficiency of endothelial progenitor cells.
21.3.3.2 Remodeling of the Resistance Arteries and Capillary Rarefaction
Changes in the structural properties of the microcirculation have been documented in CKD and ESRD. These structural modifications are characterized by remodeling of the resistance arteries and arterioles and capillary rarefaction.
Remodeling of the Small Arteries/Arterioles
Remodeling of the small arteries and arterioles is characterized by increased wall thickness, luminal narrowing, and increased wall/lumen ratio [51]. These structural features may be associated with a reduced wall cross-sectional area in eutrophic remodeling and increased wall cross-sectional area in hypertrophic remodeling [51].
Although both types of these structural alterations are often associated with hypertension, they have been reported also in CKD/ESRD [51]. In a model of uremic hypertension in rats, systemic resistance arteries (cremasteric and mesenteric) exhibited inward eutrophic remodeling while the cerebral arteries were spared [51]. Similar observations were made in normotensive rats with renal failure [52]. In these animals, intramyocardial arterioles were thickened although their BP was not elevated [52]. These observations suggest that renal functional impairment may provoke remodeling of resistance arteries even in the absence of hypertension [51–54].
Capillary Rarefaction
Capillary rarefaction defined as a reduction in the number or length of capillaries or both appears to be a histopathologic feature of advanced renal functional impairment [54–56]. In experimental animals, in addition to the intramyocardial arteriolar wall thickening, capillary rarefaction has been reported in experimental uremic animals [55]. These changes were independent of BP levels [55].
Similar histopathologic findings involving the microcirculation have been reported in patients with uremic cardiomyopathy [43].
21.4 Chronic Kidney Disease in Pediatric and Young Adults
21.4.1 Epidemiology
As in adults, the life expectancy of pediatric, adolescent, and young adult patients with CKD is significantly shortened [8, 58, 59]. Several studies have indicated that in the United States, children on dialysis live 40–60 years less than their nonrenal counterparts [8, 58, 59]. Similarly, the mortality of young adults (aged 25–34 years) on dialysis is 700-fold higher than in age-related subjects in the general population [8, 58–60]. Analysis of data from Australia and New Zealand Dialysis and Transplant Registry has also demonstrated that in all children and adolescents who were 20 years of age or younger at the initiation of renal replacement therapy, mortality rates were 30 times higher than in age-matched individuals in the general population [8, 58–60].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


