Fig. 8.1
(a, b) Routine EKG at rest. Despite correct biventricular pacing (axis directed upper right), the QRS complex is wide. Several lead configurations have been tried assessing EKG and echocardiographic findings in order to find the best stimulation without success
Report: sinus rhythm, heart rate 72 bpm with atrium-driven biventricular pacing, QRS duration 160 msec.
Despite correct biventricular pacing (axis directed upper right), the QRS complex is wide. Several lead configurations have been tried assessing EKG and echocardiographic findings in order to find the best stimulation without success.
Routine Laboratory Tests
Complete blood count: normal
Cholesterol (total, HDL, LDL) and TG: normal
Hepatic function (GOT, GPT, γ-GT, ALP, bilirubin): slight increase in transaminases
Thyroid function (TSH, FT3, FT4): normal
Renal function: creatinine 1.7 mg/dl (estimated glomerular filtration rate with the Cockcroft–Gault equation (GFR-CG) = 50 ml/min → moderate chronic kidney disease), BUN 55 mg/dl
Electrolytes: mild hyponatremia 134 mEq/l and hypokalemia 2.1 mEq/l
Fasting blood glucose: 194 m/dl (10.78 mmol/L)
HbA1c: 6.8 % (50.8 mmol/mol)
TnI-hs and CK-MB: normal
BNP: 1,100 pg/ml
Chest X-Ray
A chest X-ray was performed too (Fig. 8.2). Cardiac shadow was slightly enlarged for an increase of cardiac transverse diameter. A left-sided pleural effusion was seen obliterating the costophrenic recess associated with a bilateral hilar enlargement with widespread bronchovascular marking and interstitial pulmonary congestion was presence of right ventricular and atrial leads and coronary sinus lead.
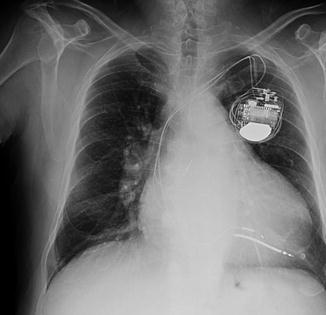

Fig. 8.2
Chest X-ray. The cardiac shadow is slightly enlarged due to an increase of cardiac transverse diameter. A left-sided pleural effusion obliterates the costophrenic recess and is associated with a bilateral hilar enlargement with widespread bronchovascular marking and interstitial pulmonary congestion. The presence of right ventricular and atrial leads and coronary sinus lead
Echocardiography
In order to complete the diagnostic process at admission, an echocardiography was performed.
The left ventricle was severely dilated (indexed left ventricular end-diastolic volume (iLVEDV) 167 ml/m2) with severe reduction of systolic function (ejection fraction measured with Simpson’s biplane method = 25 %; stroke volume = 29.4 cc; cardiac output = 2.1 l/min; cardiac index = 1 l/m2) and restrictive diastolic pattern. Moreover, filling pressures were increased (E/E’ = 16).
Right ventricle was slightly dilated and hypokinetic (basal diameter “RVD1” = 42 mm; tricuspid ring excursion “TAPSE” = 14 mm).
Severe dilatation of both atria (LA diameter M-mode = 53.5 cm; RA area A4C = 34 cm2).
No pericardial effusion.
The inferior vena cava was dilated without inspiratory collapse.
The aortic valve was trileaflet and sclerotic.
The mitral ring was dilated with moderate valvular regurgitation.
There was evidence of massive tricuspid regurgitation with pulmonary hypertension (estimated PAPs of about 60 mmHg).
Clinical Course and Medical Therapy
Furosemide: 40 mg t.i.d. (three times a day) intravenous boluses
Slow-release potassium chloride: 1,200 mg b.i.d. (bis in die) per os
Canrenoate potassium: 100 mg o.d. (once daily) intravenous boluses
Saline 0.9 % continuous infusion 40 cc/h + KCl 40 mEq
Dobutamine (250 mg/50 ml): 2 μg/kg/min continuous infusion
Dopamine (200 mg/50 ml): 2 μg/kg/min continuous infusion
Metoprolol: 25 mg b.i.d. per os
Ivabradine: 5 mg b.i.d. per os
Amiodarone: 200 mg o.d. per os
Warfarin: according to INR
Tamsulosin: 0.4 mg o.d. per os
Dutasteride: 0.5 mg o.d. per os
Pantoprazole: 20 mg o.d. per os
C-PAP therapy was administered (PEEP 7.5 mmHg, FiO2 0.5) with intermittent cycles of about 2 h.
After 48–72 h there was a significant loss of fluids supported by good diuresis. The patient reported improvement in dyspnea, and we observed a consistent reduction of ankle edema. The inotropic support was gradually discontinued. The laboratory tests showed a progressive increase of potassium values and reduced levels of BNP. The chest X-ray showed a reduced degree of pulmonary congestion, and the echocardiogram showed stable EF (0.25) associated with a small increase in cardiac index (1.3 ml/min/m2) and a slight reduction of the PAPs (50 mmHg).
Once the patient reached an acceptable grade of compensation, he was proposed for the implantation of a left ventricular assist device as destination therapy. For this purpose the patient underwent to a new coronary angiography and chest–abdomen CT with contrast which were completely normal (Fig. 8.3a, b).
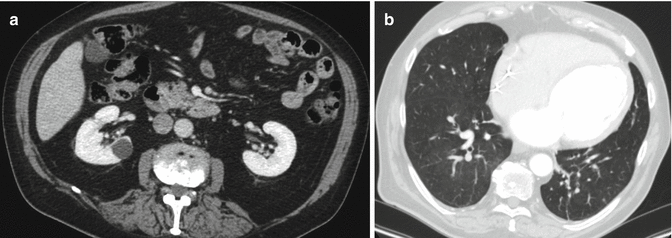

Fig. 8.3
(a, b) Chest–abdomen CT with contrast, with completely normal appearance
In order to perform a complete evaluation before the left ventricular assist device implant, we performed a right heart catheterization which showed:
Normal pressures in the right atrium, right ventricle, pulmonary artery, and wedge
Slight increase in total pulmonary resistance
Arteriolar resistance at the upper limit of normal
By the investigations carried out during the hospitalization, the patient has been judged suitable for LVAD as a destination therapy and added to the waiting list at a referral center for the implant.
Until the LVAD implant, on top therapy to chronic heart failure including loop diuretic, aldosterone antagonist, ACE inhibitor, and beta-blocker, the patient underwent cyclic intravenous inotropic therapy.
8.2 Heart Failure
Definition
Heart failure (HF) can be clinically defined as a syndrome in which patients have typical symptoms (e.g., breathlessness, ankle swelling) and signs (e.g., elevated jugular venous pressure, pulmonary crackles) resulting from an abnormality of cardiac structure or function [1].
Chronic heart failure (CHF) can be caused by several types of cardiac dysfunction and is most commonly due to left ventricular dysfunction. An isolated right ventricular (RV) dysfunction is very rare, and generally RV involvement is secondary to left ventricular (LV) dysfunction.
Demonstration of an underlying cardiac cause is essential to diagnose HF as the precise pathology determines the specific treatment used. More recently, CHF has been classified into two categories: HF due to LV dysfunction even called HF with reduced ejection fraction (HF-REF or systolic heart failure) and HF where only a diastolic dysfunction is detectable called HF with preserved ejection fraction (HF-PEF or diastolic heart failure). 1 HF-REF is the best understood type of HF in terms of pathophysiology and treatment and is the focus of this chapter.
Furthermore, HF can present either as a chronic condition or acutely, occurring de novo, or as a decompensation of CHF. The purpose of this chapter is to cover CHF, while acute heart failure is discussed in another section of this book.
Epidemiology
CHF prevalence is 1–2 % of the population, and the prevalence increases to approximately 15 % in the elderly [2]. At least half of patients with HF have a low EF, and approximately 50 % of patients with significant LV systolic dysfunction have no symptoms or signs of heart failure. HF occurs more frequently in male rather than female sex.
Etiology
The causes of CHF are listed in Table 8.1. There is a geographical variation regarding the etiology of CHF. In Western countries two-thirds are secondary to ischemic disease, and other important contributors are hypertension, valve disease, and alcohol. Rheumatic disease still remains the most common cause of CHF in the developing countries, while Chagas disease is frequent in South America.
Table 8.1
Causes of chronic heart failure
Coronary artery disease |
Hypertension |
Valve disease |
Congenital heart disease |
Infective: viral myocarditis, Chagas, HIV, Lyme disease |
Alcohol |
Toxins: anthracyclines or trastuzumab |
Deficiencies: beriberi, thiamine |
Hemochromatosis |
Idiopathic |
Familiar |
Peripartum |
Tachycardia induced |
Infiltrative states: amyloid, sarcoid, endomyocardial fibrosis, hypereosinophilic syndrome |
High output: AV fistulae, Paget’s disease |
Pathophysiology
Left ventricular dysfunction is associated with hemodynamic, autonomic, neurohumoral, and immunological changes.
The term “systolic dysfunction” refers to a decrease in myocardial contractility and consequently a decrease in cardiac output. Signs and symptoms of HF are due in part to compensatory mechanisms utilized by the body in an attempt to adjust for a primary deficit in cardiac output. Many of the processes involved in sustaining HF are maladaptive which means that they were originally designed to maintain blood pressure and vital organ perfusion.
Changes in Hemodynamics
Decrease of cardiac output leads to an increase of:
Left ventricular end-diastolic pressure
Pulmonary capillary wedge pressure
Based on the Frank–Starling law, the initial increase of left ventricular end-diastolic pressure is initially compensated by an increase of contractility, but as the increase persists, the myocardium fails and cardiac output drops.
Neurohumoral Changes
Neurohumoral adaptations, such as activation of the renin–angiotensin–aldosterone and sympathetic nervous systems by the low-output state, can contribute to maintenance of perfusion of vital organs in two ways:
Maintenance of systemic pressure by vasoconstriction, resulting in redistribution of blood flow to vital organs
Restoration of cardiac output by increasing myocardial contractility and heart rate and by expansion of the extracellular fluid volume
The principal neurohumoral systems involved in the response to HF are the sympathetic nervous system, the renin–angiotensin–aldosterone system (RAAS), and antidiuretic hormone [3, 4, 5]. One of the first responses to a decrease in cardiac output is activation of the sympathetic nervous system, resulting in both increased release and decreased uptake of norepinephrine at adrenergic nerve endings. The effects of high circulating concentrations of epinephrine and norepinephrine include:
Increase in heart rate, blood pressure, and myocardial oxygen demand
A toxic damage on the myocardium leading to cell apoptosis
A downregulation of beta-1 receptors in the heart
The decrease of cardiac output leads to a reduction of renal afferent arteriolar blood flow causing secretion of renin and, subsequently, production of angiotensinogen and angiotensin I. The angiotensin I is then converted by the ACE present in the lung to angiotensin II. There is also evidence that angiotensin II can be synthesized locally at a variety of tissue sites including the kidney, blood vessels, adrenal gland, and brain [6].
Angiotensin II increases aldosterone release, inducing systemic and renal vasoconstriction. Furthermore, angiotensin II can act directly on myocytes and in the myocardium to promote pathologic remodeling as myocyte hypertrophy, re-expression of fetal protein isoforms, myocyte apoptosis, and alterations in the interstitial matrix. Aldosterone-mediated effects are sodium and water retention and hypokalemia resulting in pulmonary and peripheral edema and increased afterload. Activation of the carotid sinus and aortic arch baroreceptors by the low cardiac output in heart failure leads to enhanced release of antidiuretic hormone and stimulation of thirst. Elevated levels of ADH may contribute to the increase in systemic vascular resistance in HF via stimulation of the V1A receptor, which is found on vascular smooth muscle cells, and also promote water retention via the V2 receptor by enhancing water reabsorption in the collecting tubules. The combination of decreased water excretion and increased water intake via thirst often leads to a fall in the plasma sodium concentration. The degree of hyponatremia is an important predictor of survival in these patients.
The Natriuretic Peptide System
The increased LV and left atrium wall stretch due to raised left ventricular end-diastolic pressure leads to secretion of the natriuretic peptide hormones. The types of natriuretic peptide hormones which circulate in high concentration in HF are:
Brain natriuretic peptides
BNP, the active peptide
NT-proBNP, the inactive N-terminal fragment
Atrial natriuretic peptides (ANP and NT-ANP)
These peptides cause:
Natriuresis
Vasodilatation
Offset in the activation of RAAS
Plasma ANP levels rise early in the course of the disease and have been used as a marker for the diagnosis of asymptomatic left ventricular dysfunction.
All these processes are responsible for sodium and water retention and a progressive depression of myocardial function. The last step of this fall is an adverse remodeling of the left ventricle involving myocyte hypertrophy, death, and fibrosis.
Diagnosis
Clinical Symptoms and Signs
The three most common symptoms and signs of HF are:
Breathlessness
Fatigue
Peripheral edema
Breathlessness is induced by exercise, and only in case of advanced heart failure, it appears at rest. Symptoms that are more specific (i.e., orthopnea and paroxysmal nocturnal dyspnea) are less common, especially in patients with milder form of HF and who are, therefore, insensitive [7, 8].
Many of the symptoms of HF are nonspecific and do not, therefore, help discriminate between HF and other problems.
Symptoms are used to assign NYHA class to patients as listed in Table 8.2.
Table 8.2
Since many of the symptoms of HF are nonspecific and do not, therefore, help discriminate between heart failure and other problems, patients are usually assigned an NYHA class
New York Heart Association functional classification based on the severity of symptoms and physical activity | |
|---|---|
Class I | No limitation of physical activity. Ordinary physical activity does not cause undue breathlessness, fatigue, or palpitations |
Class II | Slight limitation of physical activity. Comfortable at rest, but ordinary physical activity results in undue breathlessness, fatigue, or palpitations |
Class III | Marked limitation of physical activity. Comfortable at rest, but less than ordinary physical activity results in undue breathlessness, fatigue, or palpitations |
Class VI < div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |