Distribution: jaw, neck, back, arm, abdomen, left chest
Onset: at rest, during sleep, post-prandial, during mental stress
Character: sharp, stabbing, fleeting
Associated symptoms: nausea, vomiting, fatigue, dyspnea, palpitations, indigestion, sweating, syncope
Typical chest pain still occurs more frequently in women. A history of typical angina confers a predictive value of greater than 60 % for coronary artery disease (CAD) on the coronary angiogram [14]. Common atypical triggers for women include stress, rest and sleep [15]. An effective history also screens for non-cardiac causes and prevents the need for further invasive testing. Red flag diagnoses are presented in Table 9.2.
Table 9.2
Red flag diagnoses
Diagnosis | Differentiating signs and symptoms | Investigations |
|---|---|---|
Acute coronary syndrome (ACS) | Acute chest pain | Elevated biomarkers |
± dyspnea, fatigue, nausea, syncope | Ischemic ECG changes | |
Takotsubo cardiomyopathya | Acute chest pain | Mild biomarker elevation |
± dyspnea, shock | No obstruction on angiography | |
Precipitated by emotional stress | Circumferential ballooning on echo (apex, mid or base). | |
Aortic dissection | Tearing chest pain, radiation to the back | Widened mediastinum on CXR |
± aortic regurgitation, neurological deficits | Echocardiography | |
Pulse deficits | CT scan | |
Pulmonary embolism | Chest pain, may be pleuritic | V:Q scan |
± dyspnea, tachycardia, fever | CT pulmonary angiography | |
Cardiac tamponade | Chest pain | Pericardial effusion on echocardiography |
Tachycardia, hypotension, muffled heart sounds | ||
Pulsus paradoxus | ||
Tension pneumothorax | Acute dyspnea, pleuritic chest pain | Pneumothorax ± displaced mediastinum on CXR |
Reduced breath sounds, resonance to percussion, tracheal deviation | ||
Spontaneous coronary artery dissectiona | Symptoms of ACS, recent child birth | Angiography or intravascular ultrasound demonstrating true and false lumen |
Coronary vasospasma | Chest pain at rest, sometimes during sleep | ST-changes on EKG |
Younger women |
Systems Review
Women are significantly more likely than men to report multiple symptoms [16] in the setting of chest pain so it is important to conduct a thorough cardiovascular systems review, with particular emphasis on the presence of palpitations, dyspnea and fatigue. In addition, a brief screening of other systems assists both in ruling out common differentials in women, such as esophageal spasm, anxiety and pleurisy, and in as identifying further risk factors for coronary vascular disease (Table 9.3).
Table 9.3
Systems review
Systems: [17] |
Cardiovascular |
Dyspneaa |
Palpitationsa |
Peripheral edema |
Cyanosis |
Syncopea |
Diaphoresisa |
Gastroenterological |
Reflux, indigestion, esophageal spasmb |
Nausea and vomitinga |
Abdominal pain or discomfort |
Psychosocial |
Depressionb |
Anxietyb |
Somatoform disorders |
Respiratory |
Pleuritic chest pain |
Musculoskeletal |
History of traumab |
Neuropathic pain |
Swelling, deformity |
Rheumatological |
Rheumatoid arthritis, systemic lupus erythematosus, psoriatic arthritisc |
Gender-Specific Assessment
The assessment of chest pain is incomplete without a thorough obstetric and gynecological history (Table 9.4) as many of these conditions may mask or exacerbate cardiovascular disease.
Table 9.4
Obstetric/gynecological review
Menopausal status |
Risk factor for CAD, compounded by effects of ageing |
Hormone replacement therapy (HRT) as a cardiovascular protection agent in post-menopausal women is controversial, with the HERS trial finding no benefit to this regime, with an increased rate of venous thromboembolic events (VTE) and MI in the early stages of therapy. |
Pre-eclampsia |
Pregnancy is often seen as a physiological stress test for cardiovascular disease, and is also a time when women first undergo blood pressure and blood glucose monitoring, thus making it an essential part of a cardiovascular risk assessment in women [21]. |
Pre-eclampsia is a marker of maternal predisposition to vascular disease, particularly at a younger age [24] |
Gestational diabetes |
Increased risk of cardiovascular disease and type 2 diabetes [25]. |
Polycystic Ovarian Syndrome (PCOS) |
Increased cardiovascular risk, as well as increasing the risk of co-morbidities such as insulin resistance, obesity and hyperlipidemia [26]. |
Women with PCOS are at a higher risk than their age-matched counterparts and require earlier screening for CVD |
Oral contraceptive pill (OCP) |
While some meta-analyses have indicated a higher risk of MI attributable to increased thrombogenicity, the overall attributable risk is low, and thus considered safe [29]. |
Risk Factor Assessment and Risk Stratification
Women present with a more extensive risk factor profile at their index event than men, due in part to an older age at presentation, but are less likely to have prior history of IHD or revascularization [30]. Table 9.5 shows the risk factors for CAD in women. The presence of cardiovascular risk factors is a stronger predictor of CAD in women than in men [12].
Table 9.5
Risk stratification tool
Moderate risk criteria | High risk criteria |
|---|---|
One or more of the following Smoking Poor diet Sedentary lifestyle Obesity (particularly central) Family history of premature CVD Hypertension Dyslipidemia (particularly hypertriglyceridemia [31]) Subclinical vascular disease Metabolic syndrome Poor exercise capacity on ETT | Known coronary artery disease Other arterial disease, including Cerebrovascular disease Peripheral arterial disease Abdominal aortic aneurysm Chronic renal impairment Diabetes mellitus 10-year Framingham Global Risk >20 % |
The Framingham risk estimation score, which is the current standard of risk stratification, is a poor predictor of subclinical disease in women, and has the tendency to misclassify those who would benefit from investigation as low risk. As a result, these women potentially evade adequate assessment and thus the threshold for investigation should be lower [33]. Accuracy may be improved with the use of imaging assessment, such as the coronary artery calcium score, which is discussed later in this chapter.
Diabetes mellitus is the strongest independent predictor of CAD in women, to a much greater extent than in men. The presence of DM increases the chance of developing CAD three-fold [34, 35]. Similarly, the presence of concurrent peripheral vascular disease is equivalent to an existing diagnosis of CAD. The presence of one or more major risk factor should warrant consideration of further investigation.
Investigations
Despite best practice guidelines, women are significantly less likely to undergo exercise ECG stress testing and coronary angiography than their male counterparts [36]. Not surprisingly, women presenting with stable angina have an increased risk of 1-year mortality, which highlights the need for a shift in the paradigm in the assessment and treatment of women with chest pain. Female gender, coupled with age <55, functions as an independent risk factor for missed myocardial infarction [37].
Several factors complicate the investigation of chest pain in women [38]. Anatomically, women have smaller body surface area, narrower coronary arteries and a higher frequency of abnormal plaque morphology, rendering invasive procedures and assessment more difficult [39]. Risk stratification standards in women remain suboptimal, as evidenced by the higher incidence of women undergoing angiography only to find normal coronary anatomy, highlighting the need for improvement in triage and investigation [11]. Figure 9.1 shows investigations for women presenting with chest pain.
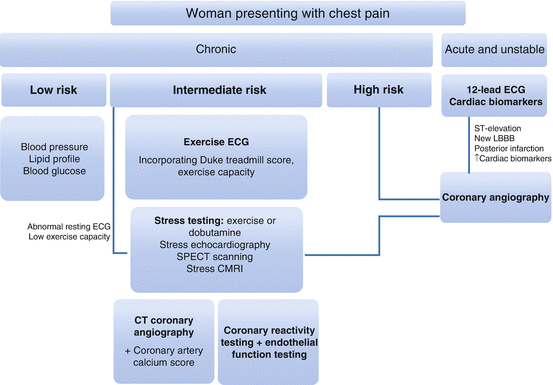

Fig. 9.1
Investigations for women presenting with chest pain
Acute Chest Pain
ECG
The standard 12-lead ECG is integral to the initial evaluation and triage of patients with suspected ACS. Ideally, this needs to be performed within 10 min of presentation, but this occurs less frequently in women [40, 41]. Discrepancies in the ECG seen in women include a prolonged QT interval, reduced QRS amplitude and duration, and reduced baseline ST deviation. In particular, these parameters reduce the efficacy of diagnostic criteria for left ventricular hypertrophy, and despite the development of gender specific criteria, this condition continues to be under-diagnosed [42]. For STEMI, current guidelines advocate a lower threshold for diagnosis of ST elevation in women in leads V2-3, with ≥1.5 mm elevation signifying STEMI, as opposed to ≥2 mm in men [43, 44].
Potentially, there may be a role for echocardiography in women presenting with suspected IHD in the absence of diagnostic ECG changes or an atypical pattern of symptomatology.
Cardiac Biomarkers
A multi-marker approach is advocated in the assessment of women with chest pain due to gender discrepancies in reference range and specific biomarker expression, with women less likely to present with troponin elevations than men [45]. The TACTICS-TIMI 18 trial found that in women with non-ST-segment acute coronary syndromes were more likely to have elevated BNP and hs-CRP than men, who were more likely to present with elevated troponin and CK-MB [46]. In addition, CRP is related to micro-vascular coronary disease, a common but atypical cause of ischemic chest pain in women, and may be associated with the post-menopausal drop in estrogen [47]. It may also play a role as a risk marker in women [48]. Advantages and disadvantages of imaging modalities are presented in Table 9.6.
Table 9.6
Advantages and disadvantages of imaging modalities
Modality | Advantages | Disadvantages |
|---|---|---|
Exercise ECG | Tests exercise capacity Prognostic information | Lower sensitivity and specificity in women due to baseline ST variation |
Stress Echo | Higher sensitivity and specificity than exercise ECG Provides information on wall motion abnormality, LVEF, structural abnormalities No radiation, quick procedure Good negative predictive value | Operator variability |
Coronary artery CT | Non-invasive Identifies non-obstructive and subclinical CAD (plaque burden) Prognostic indicator +++ sensitivity and specificity | Radiation exposure Good negative predictive value Limited availability |
Cardiac MRI | Structural and functional assessment Sub-endocardial perfusion ++ sensitivity and specificity | Cost Patient discomfort Operator variability |
SPECT | High sensitivity Risk stratification tool Less variation between operators | Radiation exposure Breast attenuation Smaller heart Poor detection of multi-vessel and micro-vascular disease |
Coronary angiography | Gold standard for CAD diagnosis Assessment of structure and function Intervention can be performed simultaneously | May miss extra-luminal plaque, microvascular disease Costly Invasive procedure, patient discomfort, radiation |
Coronary reactivity testing | Diagnoses endothelial dysfunction and micro-vascular disease | High rate of inconclusive results Risk of coronary artery dissection |
Chronic Chest Pain
Exercise ECG Stress Test
The exercise ECG test (ETT) is considered the first line non-invasive investigation for patients with a moderate risk of CAD and a normal baseline ECG [49]. ETT provides ischemic provocation in the form of exercise and allows interpretation of corresponding ECG abnormalities. However, there are clear gender discrepancies in the accuracy of exercise ECG testing between men and women. The sensitivity and specificity of ETT in women is just 61 % and 70 %, compared to 72 % and 77 % in men, respectively [50]. Critically, the low specificity gives rise to almost 30 % of women receiving a false negative diagnosis, indicating the clear need for improvement in diagnostic strategy. Gender variation in the accuracy of ETT is thought to be due to the baseline ST abnormalities seen in women, lower functional capacity, lower voltage and the impact of fluctuating estrogen levels on electrical recording [51, 52].
The ETT can be of value in symptomatic women when interpreted in conjunction with the Duke treadmill score [53], shown below (see Table 9.7). Exercise time is measured in minutes and the ST deviation is the absolute distance from baseline in any lead except aVR.
Table 9.7
Interpretation of Duke treadmill score
Risk | Score | Action |
|---|---|---|
Low | ≥5 | Preventative measures only |
Moderate | 5 to −11 | Cardiac imaging ± coronary angiography |
High | ≤−11 | Coronary angiography |
Also of value is the ability of the ETT to provide information on exercise capacity, which is a strong prognostic indicator in CAD. Women are considered high risk if they are incapable of performing more than five metabolic equivalents of graded exercise [54]. Heart rate recovery might also provide valuable information in women. Recovery of resting heart rate within 1–2 min post-ETT has good prognostic power [55].
Stress Echocardiography
Stress echocardiography is a highly effective non-invasive means for assessing and stratifying symptomatic women with intermediate risk of CAD. In addition, it provides the added benefit of structural assessment which allows identification of localized ventricular dysfunction, valvular disease and wall motion abnormalities when confronted with ischemic stress [56] in the form of either exercise or dobutamine. Wall motion abnormalities are an early indication of ischemia. Dobutamine stress echocardiography is recommended in women incapable of exercising regardless of baseline ECG. Stress echocardiography has a higher specificity and sensitivity than stress ECG, with a combined accuracy of roughly 85 % [49, 57–59]. The efficacy of stress echo is not gender specific. The higher specificity allows for lower rates of false positives and therefore reduces the rate of unnecessary angiography [60].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


