Category
Syndrome
Key features
Cardiac
Stable angina
Precipitated by exertion/relieved by rest
Acute coronary syndromes
Sudden onset/duration >20 min. Associated with dyspnoea and autonomic symptoms
Pericarditis
Sharp, pleuritic. Relieved by leaning forwards
Respiratory
Pneumothorax
Unilateral. Pleuritic. Sudden onset
Pleuritis/pneumonia
Pleuritic/infective symptoms
Vascular
Aortic dissection
Very severe/radiating to the back
Pulmonary embolism
Pleuritic/dyspnoea
Gastrointestinal
Gastro-oesophageal reflux
Following large meal/relieved by antacid
Peptic ulcer disease
Relieved by antacid
Pancreatitis
Intense epigastric/substernal
Gallbladder disease
Usually following fatty meal
Musculoskeletal
Costochondritis
Reproduced on palpation
Trauma
History of trauma
Neurological
Cervical spine pathology
Reproduced by neck movement
Infectious
Herpes zoster
Burning/dermatomal distribution
Psychological
Panic attack
Hyperventilation
Initial Assessment of the Patient
In the acute setting, initial assessment may take place prior to physician contact, with paramedics or nurses triaging individuals. A 12-lead electrocardiogram (ECG) should be recorded and assessed at the earliest possible opportunity, preferably within 10 min, in all patients who present with ongoing chest pain of suspected cardiac origin. The priority is to address whether the patient is clinically stable or at risk of a life-threatening condition such as ACS, aortic dissection or pulmonary embolism. If not critically ill, a decision regarding urgency for further investigation must be made by risk-stratifying patients. Patients who display ST segment elevation on their ECG must be triaged as critical priorities and directed to specialist cardiology centres with a view to cardiac catheterisation as an emergency.
The diagnosis and assessment of a patient with suspected stable angina involves a comprehensive clinical evaluation, including identifying significant dyslipidaemia, hyperglycaemia or other biochemical risk factors and specific cardiac investigations. These investigations may be used to confirm the diagnosis of ischaemia, to identify or exclude associated conditions or precipitating factors, assist in risk stratifying patients and to evaluate the efficacy of treatment (Fig. 1.1).
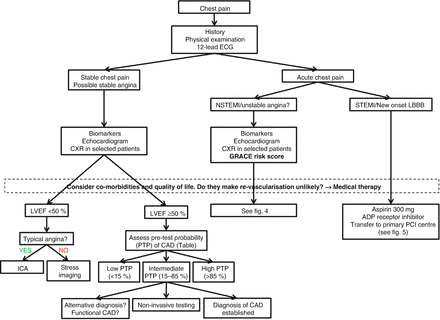

Figure 1.1
Initial diagnostic management of patients presenting with chest pain. ADP adenosine diphosphate, ICA invasive coronary angiography, LVEF left ventricular ejection fraction
History
The classic manifestation of pain that is caused by myocardial ischaemia is angina. This may be described amongst other things as dull discomfort, crushing sensation, restriction, “band-like” sensation or throat tightness. The discomfort is classically brought on by physical or in some cases emotional stress and may radiate into the jaw, one or both shoulders or arms (though not to fingertips).
A description of central chest pain that is brought on by exercise and relieved by rest or by the use of glyceryl trinitrate (GTN) is thought to constitute typical angina. By contrast the following descriptions of pain make the diagnosis of angina less likely:
(a)
pain that is primarily pleuritic
(b)
pain that is entirely in the abdomen
(c)
pain that can be localized by the patient with the use of one finger
(d)
pain that lasts for a few seconds or pain that persists for many hours or days
(e)
pain that can be reproduced by movement or palpation of the chest or of the arms
In general, patients with symptoms of chest discomfort, dyspnoea or dizziness that are clearly related to exertion should be referred for further assessment with regards to underlying coronary artery disease (CAD).
Risk Factors
The main risk factors for ischaemic heart disease are well described in various population studies. Non-modifiable risk factors include increasing age and male gender; male patients over the age of 70 have over 90 % probability of underlying CAD even when presenting with atypical pain (Table 1.2).
Table 1.2
Clinical pre-test probability in patients with stable chest pain symptoms
Typical angina | Atypical angina | Non-anginal pain | ||||
|---|---|---|---|---|---|---|
Age (yrs) | Male | Female | Male | Female | Male | Female |
30–39 | 59 | 28 | 29 | 10 | 18 | 5 |
40–49 | 69 | 37 | 38 | 14 | 25 | 8 |
50–59 | 77 | 47 | 49 | 20 | 34 | 12 |
60–69 | 84 | 58 | 59 | 28 | 44 | 17 |
70–79 | 89 | 68 | 69 | 37 | 54 | 24 |
>80 | 93 | 76 | 78 | 47 | 65 | 32 |
Amongst the modifiable risk factors, diabetes mellitus, smoking status and hyperlipidaemia confer a higher predictive value than hypertension. Familial premature CAD (below age 55 years in a male non-smoker, below 65 years in a female), usually occurs in the context of other risk factors such as familial dyslipidaemia and thus, on its own, does not confer as high a predictive value as previously thought.
It should be noted that this risk factor profiling applies to patients with no previous diagnosis of CAD, i.e. only at the time of index presentation. Any patient with a prior myocardial infarction (MI), demonstrable CAD on coronary angiography or prior coronary revascularization has an established diagnosis of CAD. Thus, chest discomfort in such patients should be thoroughly evaluated. Risk factor modification in such patients is important for their prognosis and prevention of recurrent cardiovascular events.
Physical Examination
A thorough examination of the cardiovascular and respiratory system should be performed in all patients. Although there are no pathognomonic physical signs of CAD, a physical examination may reveal signs that point towards alternative diagnoses. These may include signs of:
(i)
systemic conditions/illnesses (e.g. sepsis, anaemia, thyroid disease, generalized malignancy)
(ii)
other heart disease (e.g. murmur of aortic stenosis or hypertrophic cardiomyopathy, friction rub of pericarditis)
(iii)
vascular disease (e.g. difference in arm blood pressures indicating aortic dissection, signs of pulmonary hypertension in pulmonary embolism)
(iv)
respiratory disease (e.g. signs of pneumonia, pleural rub, signs of pneumothorax)
Investigations
ECG
A 12-lead ECG should be obtained and interpreted at presentation. The following criteria constitute acute transmural ischaemia, consistent with the diagnosis of ST elevation myocardial infarction (STEMI) in a patient presenting with symptoms of chest pain and should prompt urgent specialist referral:
ST elevation of ≥2 mm in at least two contiguous precordial leads (V1-V6)
ST elevation of ≥1 mm in at least two limb leads of same territory (lateral: I, aVL; inferior: II, III, aVF)
Left bundle branch block (LBBB) that is not known to be old
ST-depression in leads V1-V3 with associated dominant R-wave (suggestive of isolated posterior STEMI) (Fig. 1.2)
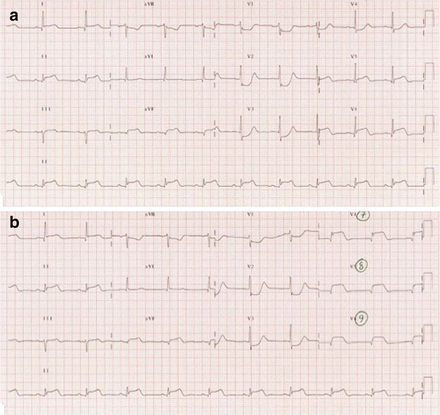
Figure 1.2
(a) ECG demonstrating an infero-postero-lateral STEMI in a patient with acute chest pain. Note the presence of ST-depression in leads V1-V3 with associated dominant R-wave. (b) Placing leads V4-V6 posteriorly (V7-V9) unmasks the posterior ST-elevation
ST-segment elevation in lead aVR, particularly when associated with precordial lead ST depression is suggestive of left main stem ischaemia (Fig. 1.3)
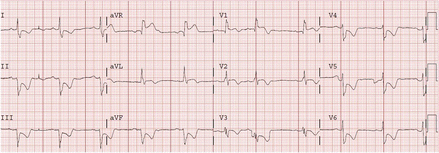
Figure 1.3
ECG demonstrating ST-segment elevation in lead aVR, with associated ST depression in the precordial leads, suggestive of left main stem ischaemia
The presence of a ventricular paced rhythm and LBBB make interpretation of the ECG for evidence of acute ischaemia challenging. In such cases, patients with ongoing symptoms suggestive of myocardial ischaemia, such as persistent chest pain, autonomic symptoms (sweating and nausea), signs of acute heart failure or impending shock also deserve prompt specialist assessment. It’s also important to remember that a normal ECG does not rule out an acute coronary event!
Patients with ECG abnormalities that do not fall into any of the categories detailed above are not thought to benefit from early reperfusion therapy. However patients with clearly dynamic ECG changes (dynamic ST depression that occurs during episodes of chest pain or progressive T-wave inversion and deepening) may be considered for early coronary angiography.
ECG rhythm monitoring should be initiated as soon as possible in all patients with suspected STEMI and continue for at least 24 h after successful reperfusion therapy due to the risk of acute ischaemic or early post-MI ventricular arrhythmia.
Myocardial Biomarkers
The use of biomarkers in the diagnosis of myocardial ischaemia has changed considerably over recent years. The diagnosis of myocardial infarction was initially reliant on assays of creatine kinase (CK), its iso-enzyme (CK-MB) and myoglobin. Although useful, they were limited by low sensitivity and specificity. The advent of troponin assays allowed for greater diagnostic accuracy and thus troponin is now the biomarker of choice for detecting myocardial damage. In patients with a myocardial infarction, levels may be elevated as early as 4 h after the event and can remain elevated for up to 2 weeks. A troponin assay may therefore also be of value in the assessment of patients who present late. High sensitivity troponin assays provide even greater sensitivity and by using serial samples, for example 3 h apart, the sensitivity for diagnosing MI approaches 100 %. Current guidance advocates a troponin sampling at admission and repeat measurement between 10 and 12 h after the onset of symptoms. This provides the opportunity to assess the pattern of change. The progression from the first to second sample is important as it may help to differentiate acute infarction from chronic myocardial necrosis.
It is important to note that troponin should not be used as the sole investigation to diagnose or exclude ACS. The history, clinical findings and ECG remain the cornerstone of the diagnostic process. Troponin levels may be elevated in response to other pathologies (Table 1.3) and one must be prepared to consider an alternative diagnosis as suggested by the history and clinical examination findings.




Table 1.3
Causes of raised troponin levels (apart from ACS)
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


