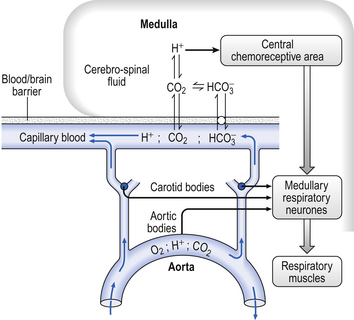
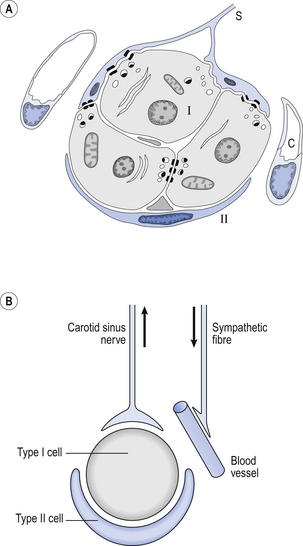
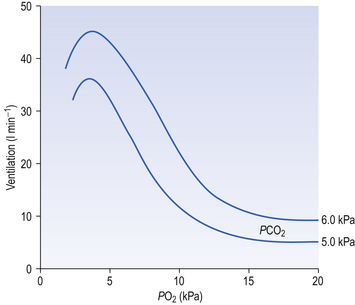
9 It is rare for excess CO2 or lack of O2 to occur alone: they usually occur together, and the whole chemoreceptor system is shown schematically in Figure 9.1. The term for a lack of oxygen in any gas mixture or solution is hypoxia. Lack of O2 in arterial blood is termed hypoxaemia. Total absence of O2 is anoxia. It is very easy to change the amount of a gas in the arterial blood by utilizing the powerful gas-transporting properties of the lungs. Simply giving a subject a gas mixture to breathe will result in his or her arterial blood taking on the composition of that gas mixture within remarkably few breaths. The rate at which equilibrium is reached depends on the solubility of the gas in body fluids, and this has important consequences in anaesthesia. However, for the gases we are concerned with here equilibrium is approached within a few dozen breaths. The chemoreceptors that sense lack of arterial O2 are the carotid bodies and the aortic bodies. In humans it is the carotid bodies that are mainly responsible for the respiratory response. They are small (5.0 mm diameter) nodules of glomus tissue (Latin glomerus, a skein or ball of thread, i.e. a knot of capillaries) situated near the bifurcation of each common carotid artery. Unlike the carotid bodies, which mainly respond to Pao2, the aortic bodies are stimulated by reductions in arterial O2 content, e.g. carbon monoxide poisoning and anaemia affect them more. So it seems that the aortic bodies are sensitive to the total amount of O2 delivered to them, and the carotid bodies are sensitive to Pao2. The carotid bodies are situated close to the baroreceptor region of the carotid arteries, which help to regulate blood pressure, and are frequently confused with them. The carotid bodies are not baroreceptors. The function of the carotid bodies is related to their unusual structure. They have an extremely high metabolic rate (about three times that of the brain) but their rate of perfusion by blood from the carotid arteries is even higher: 10 times that which would be expected. This blood flows through capillaries (Fig. 9.2A and B) which surround the sensory elements (the glomus or type I cells) that monitor blood Po2. The type I cells seem to be supported by type II (sustentacular) cells, whose function is still not clear. The type I cells send their information to the brain via the carotid sinus nerve, a branch of the glossopharyngeal nerve (Fig. 9.2A and B), which also provides them with sympathetic and parasympathetic innervation. A separate supply of sympathetic fibres from the nearby superior cervical ganglion innervates the carotid bodies’ blood vessels. 1. neural influences on the glomus cells themselves 2. neural influences on the blood vessels supplying the glomus cells. Activity in the carotid bodies is measured experimentally as the frequency of discharge of action potentials in the carotid sinus nerve. Increased activity expresses itself in the whole animal as an increase in ventilation. Hypoxia stimulates peripheral chemoreceptors, which is unusual, as the activity of almost all other organs is depressed by it. The effect of decreasing a subject’s arterial Po2 by giving them increasingly hypoxic gas to breathe is shown in Figure 9.3. 1. Chemoreceptors have a very high metabolic rate and so rapidly use up O2 supplied to them. 2. They have a very high blood flow, gram for gram 40 times that of the brain. 3. Pao2 must be reduced considerably before there is stimulation of breathing, but then the increase is large. 4. Increasing Pao2 above normal (13 kPa) by inhaling O2-rich mixtures only produces a small reduction in breathing by depressing chemoreceptor activity. 5. Increasing arterial [H+] does not have a great effect on central chemoreceptors but stimulates peripheral chemoreceptors. 6. Peripheral chemoreceptors are much less sensitive to increases in Paco2
CHEMICAL CONTROL OF BREATHING
Introduction
Oxygen lack
Histology, embryology and anatomy of the carotid bodies
Hypoxic stimulation
Hypercapnic stimulation
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


CHEMICAL CONTROL OF BREATHING