Chapter 4
Central Venous Catheters: Materials, Designs, and Selection
Currently, a wide array of central venous access devices is available, for a variety of indications, and in a multitude of sizes, shapes, lengths, and configurations to meet the demands of current medical practice.1 Despite their apparent differences, all central venous access devices are alike in that they are placed within the central venous circulation, typically in the superior vena cava (SVC), inferior vena cava (IVC), or right atrium (RA).
CATHETER MATERIAL
Virtually all current central venous catheters (particularly long-term catheters) are made of silicone rubber or polyurethane. These two materials have different properties and handling characteristics. Intravascularly placed silicone rubber has been used safely since the 1970s. Silicone is a soft, biocompatible material that has been placed within the vascular system by open cutdown for many years. This soft material, which has a high coefficient of friction, makes manipulations with standard stainless steel guidewires extremely difficult. These features, combined with nontapered tips, require the use of peel-away sheaths for percutaneous insertions. The difficulties in guidewire manipulation were minimized by the introduction of relatively stiff hydrophilic guidewires, which now are used routinely for catheter placement. Although silicone typically is used in long-term devices, temporary or short-term devices also currently are made of silicone. These temporary silicone devices have tapered tips and stiffening cannulas for percutaneous insertion without the use of hydrophilic guidewires or peel-away sheaths.
Polyurethane is a newer material that is stronger and stiffer than silicone. The stronger material allows thinner walls and larger lumen diameter while maintaining the same outer diameter of the catheter. A polyurethane catheter may have a comparable inner-diameter lumen with a smaller outer diameter. Polyurethane has a lower coefficient of friction and can be used with conventional stainless steel guidewires. A large polyurethane catheter with nontapered tips still may require peel-away sheaths; however, over-the-wire insertions of smaller polyurethane catheters are possible. Although the polyurethane material is stiff, it softens within the body, making it suitable for long-term use within the vascular system.
TIP CONFIGURATION
Catheters are available in one of three basic tip configurations: end-hole, valved tip, and staggered tip2,3 (Fig. 4–1). End-hole catheters are the standard tip design that can be trimmed at the tip to fit the patient’s anatomy without changing the design. Single-, dual-, or triple-lumen catheters are available with end-hole design. The valvedtip catheter has a closed blunt tip with valved slits just proximal to the tip. These slits allow blood to be withdrawn and solutions to be infused, but they will not allow blood to enter the lumen when not in use.4 Single- or dual-lumen catheters are available with the valved-tip configuration. The advantage over the simple end-hole catheter design is that the valved-tip catheter does not require routine heparinization to prevent catheter thrombosis. Because of the valved tip, however, these catheters cannot be trimmed at the tip during placement. Therefore, to modify the catheter length to fit anatomy, valved-tip catheters usually have a removable connection, which allows the catheter to be trimmed at the hub. Staggered-tip, dual-lumen catheters are specially designed for therapies that require rapid simultaneous aspiration and infusion with limited admixture (e.g., pheresis, hemodialysis).5 These catheters cannot be trimmed at the tip because it would defeat the purpose of staggered configuration. Although available with removable hubs, these catheters more commonly have fixed hubs made in a variety of lengths. In practice, fixed-length, staggered-tip catheters can be modified at the tip by trimming and recreating the staggered configuration, but this would alter the volume of the intracatheter heparinization solution needed and is not recommended.
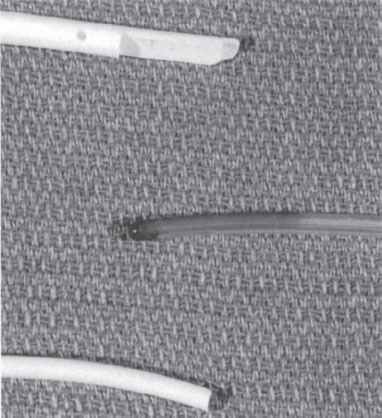
Figure 4–1 Tip configurations of central catheters. Top: Staggered tip. Middle: Valved tip. Bottom: End-hole tip.
CENTRAL VENOUS ACCESS DEVICES
Central venous access devices can be classified initially into two categories: (1) short-term (temporary) and (2) long-term (permanent) devices. The latter can be further subdivided into (1) peripherally inserted central catheters (PICCs); (2) chest-wall external catheters, which can be either tunneled or nontunneled; and (3) subcutaneous ports, which can be implanted either in the chest wall or in the extremities.
Short-Term Central Venous Catheters
These catheters usually are placed at the bedside when immediate central access is needed. Most commonly, these catheters are made of polyurethane and are available with single, dual, or triple lumen with tapered end-hole tips. Sizes range from 5 to 14 French (F). They are inserted with standard Seldinger over-the-wire techniques and are intended for short-term (days to weeks) in-hospital use. More recently, 11.5 and 14 F silicone, dual-lumen pheresis/hemodialysis catheters have become available; these have a tapered end-hole and a stiffening cannula to facilitate over-the-wire insertion.
Long-Term Central Venous Catheters
Peripherally Inserted Central Catheters
As the name indicates, PICCs are inserted through a peripheral vein of the upper extremity (basilic, cephalic, brachial, antecubital), and the tip is placed in the central circulation6 (Fig. 4–2). It is therefore a true central venous catheter. PICCs are available in 2 to 7 F with single or dual lumen. PICCs are external, nontunneled catheters and require tape or suture fixation. These catheters are convenient for home use for an intermediate period—from several weeks to 3 to 6 months. Although PICCs can remain functional for longer than 6 months, their small diameter and fragility somewhat limit their longevity.1 Advantages of the PICC include a lower procedural complication rate, patient preference, and the potential of cost-effective bedside placement by special nursing personnel. Disadvantages are their small diameter (restricted flows, fragility, poor radiopaque quality) and the need for an intact upper-extremity venous anatomy. Their small caliber also may lead to less reliable transfusion and phlebotomy.
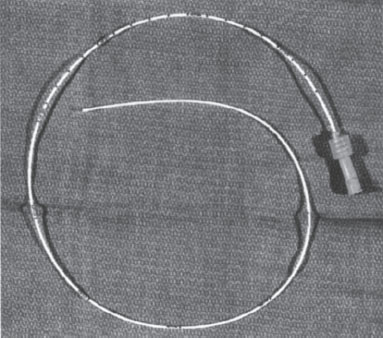
Figure 4–2 Peripherally inserted central catheter.
Silicone and polyurethane PICCs can tolerate flow rates ranging between 0.4 and 7.0 mL per second and 0.6 to 10.2 mL per second, respectively. The 5 F silicone PICC and the 4 and 5 F polyurethane PICCs routinely tolerate flows greater than 4 mL per second.7
Chest-Wall External Catheters
Nontunneled These catheters usually are made of silicone, are nontapered, and are intended for intermediate-length home therapy (weeks to months). Their longevity is similar to that of PICCs, but they are placed via the central veins.8 They are available in single-lumen 5 F and duallumen 7 F varieties (Hohn Catheter, Bard Access Devices, Salt Lake City, UT) (Fig. 4–3). Because they are made of silicone and are nontapered, insertion is more difficult than with conventional acute-care central catheters. Stabilization is with tape or suture.
Tunneled Tunneled external chest wall catheters are the “traditional” and initial long-term central venous access devices. They are available with single, dual, or triple lumen; made of silicone or polyurethane material; and with end-hole, valved-tip, or staggered-tip configurations2,3 (Fig. 4–4). Sizes range from 3.5 to 21 F for use in the pediatric population and adult hemodialysis populations, respectively. The devices have a tissue ingrowth cuff attached to the shaft that is positioned within the subcutaneous tunnel, which is made between the original venous access puncture site and the catheter exit site.9 The subcutaneous tunnel provides long-term catheter stabilization and protection from infection. The cuff incites a desmoplastic tissue response within 4 to 6 weeks that stabilizes the catheter, allowing long-term use of the device, intended for months to years. Some catheters are available with an “antimicrobial” cuff (Vitacuff; Vitaphore, Menlo Park, CA) that is positioned just proximal (closer to the skin exit site) to the tissue ingrowth cuff.10 This second cuff is intended as a temporary stabilizer and antimicrobial barrier. Some recent evidence suggests that this silver-impregnated cuff causes a local cytotoxic effect on fibroblasts and actually may delay fibrous tissue ingrowth.11
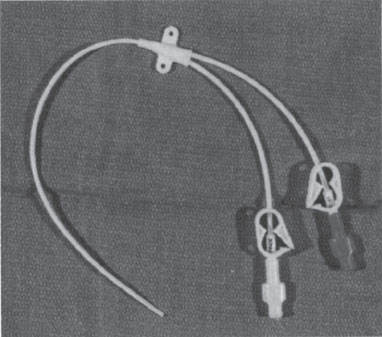
Figure 4–3 Dual-lumen nontunneled Hohn catheter.



