Catheterization Laboratory Imaging and Functional Assessment
Over the last two decades, interventional cardiology has rapidly expanded in the field of coronary and noncoronary structural interventions such as valve repair, transcatheter valve replacement, septal defect closure, etc. Coronary angiography is the gold standard for assessing coronary anatomy; however, sometimes it does not provide information about the physiologic or functional significance of a stenotic lesion, and neither does it adequately assess the severity and extent of atherosclerotic plaque in the vessel wall. Fractional flow reserve (FFR) has emerged as an excellent tool for assessing the physiologic impact of a lesion, and intracoronary imaging with intravascular ultrasound (IVUS) and optical coherence tomography (OCT) have shown promise in assessing the atherosclerotic plaque and the response of the coronary vessel wall to percutaneous coronary intervention (PCI). Integration of information from various imaging modalities such as fluoroscopy, cineangiography, echocardiography, and computed tomography (CT) is critical for safety and success of structural cardiac interventions. In this chapter, we first highlight the role of functional imaging in the catheterization laboratory by means of FFR and the role of intracoronary imaging with IVUS and OCT and then discuss structural cardiac anatomy using various imaging techniques and their utility in guiding different structural interventions.
PHYSIOLOGIC ASSESSMENT OF CORONARY ARTERY DISEASE IN THE CATHETERIZATION LABORATORY
The goals of treatment in patients with coronary artery disease (CAD) are reduction in symptoms and risk of myocardial infarction (MI) and improvement in survival. The randomized prospective COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial showed that revascularization with PCI does not reduce the risk of MI or improve survival in patients with stable CAD when added to optimal medical therapy (OMT).1 Presence of myocardial ischemia is a risk factor for adverse clinical outcome in patients with CAD, and concurrently, PCI was shown to reduce major adverse events in the subset of COURAGE population with significant ischemia on stress testing.2 The observed lack of benefit of PCI in all comers with stable CAD is likely due to the lack of correlation of severity of coronary stenosis, as assessed by coronary angiography, with the degree of ischemia or hemodynamic significance of coronary stenosis.3 In addition, PCI has occasional complications, which can affect long-term outcomes. Coronary angiography provides a two-dimensional (2-D) image of the three-dimensional (3-D) vessel lumen, which coupled with vessel overlap, tortuosity and eccentricity of lesions, often makes it very difficult for the angiographer to assess the functional or physiologic impact of a lesion despite multiple views. This can be assessed most accurately by means of a noninvasive stress test or an invasive physiologic test that can be performed in the catheterization laboratory, namely FFR.
Fractional Flow Reserve
FFR is defined as the ratio of maximal blood flow in a stenotic artery to normal maximal flow.4 It can be measured during coronary angiography by passing a 0.014-inch pressure sensor angioplasty wire through a guiding catheter into the coronary artery. The wire’s pressure signal is first matched with the aortic pressure (Pa) and then the lesion in question is crossed with the wire. Coronary hyperemia is then induced, usually with intravenous adenosine. The pressure in the coronary artery distal to the stenotic lesion (Pd) and Pa are continuously recorded, and FFR is calculated as the ratio Pd/Pa at maximal hyperemia (Fig. 46.1).
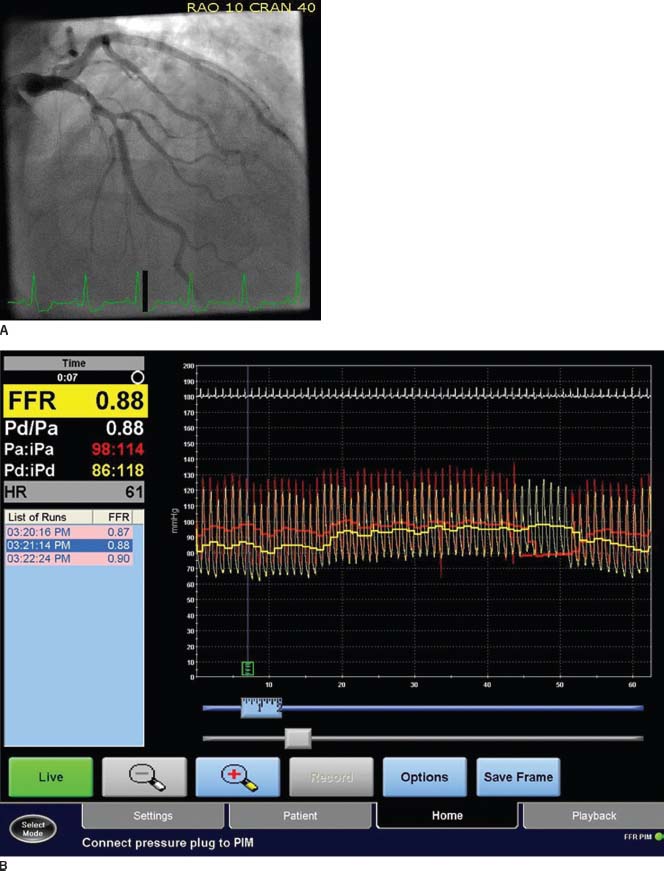
FIGURE 46.1 A: Coronary angiogram demonstrating intermediate severity lesion (50% to 60% stenosis) in the mid-LAD. B: FFR demonstrating a value of 0.88 indicating hemodynamically insignificant stenosis.
 FFR in a normal coronary artery equals 1.0. An FFR value of 0.80 or less indicates hemodynamically significant coronary stenosis with an accuracy of >90%.5
FFR in a normal coronary artery equals 1.0. An FFR value of 0.80 or less indicates hemodynamically significant coronary stenosis with an accuracy of >90%.5
In contrast to noninvasive myocardial perfusion imaging studies, FFR is a vessel-specific index of ischemia and hence more specific and has better spatial resolution. In the DEFER study, 325 patients underwent FFR of an intermediate coronary lesion prior to planned PCI. Patients with FFR ≥ 0.75 were randomized to deferral of PCI (Defer group, n = 91) or performance of PCI (Perform group, n = 90).6 If FFR was ≤0.75, patients PCI was performed as planned (Reference group; n = 144). At 5 years of followup, the event-free survival was similar in the Defer and Perform groups (80% vs. 73%, p = 0.52). In addition, the composite rate of death and MI was not different in the Defer and Perform groups (3.3% vs. 7.9%, p = 0.21).6 Similarly, in patients with multivessel CAD, nonrandomized studies have shown that FFR-guided PCI is associated with favorable outcomes and lower cost when compared to angiographically guided PCI without a physiologic assessment by FFR.7,8
These studies led to the large, prospective, multicenter, randomized FAME trial (FFR versus Angiography in Multivessel Evaluation), to compare FFR-guided PCI compared to conventional angiographically guided PCI in patients with multivessel CAD.9
 In the FAME study, 1,005 patients with multivessel CAD were randomly assigned to undergo PCI with drug-eluting stent (DES), guided by angiography alone or PCI guided by an abnormal FFR ≤ 0.80. The mean SYNTAX score was 14.5 in each group, indicating low-intermediate risk patients.
In the FAME study, 1,005 patients with multivessel CAD were randomly assigned to undergo PCI with drug-eluting stent (DES), guided by angiography alone or PCI guided by an abnormal FFR ≤ 0.80. The mean SYNTAX score was 14.5 in each group, indicating low-intermediate risk patients.
 The 1-year combined primary endpoint of death, MI, and repeat revascularization was lower at 13.2% in the FFR-guided PCI group compared to 18.3% in the angiography-guided PCI group (p = 0.02). In addition, the FFR-guided PCI group had less death or MI (7.3% vs. 11.1%, p = 0.04), fewer stents used per patient (1.9 ± 1.3 vs. 2.7 ± 1.2, p < 0.001), less use of contrast (272 mL vs. 302 mL, p < 0.001) and lower procedural cost ($5,332 vs. $6,007, p < 0.001).
The 1-year combined primary endpoint of death, MI, and repeat revascularization was lower at 13.2% in the FFR-guided PCI group compared to 18.3% in the angiography-guided PCI group (p = 0.02). In addition, the FFR-guided PCI group had less death or MI (7.3% vs. 11.1%, p = 0.04), fewer stents used per patient (1.9 ± 1.3 vs. 2.7 ± 1.2, p < 0.001), less use of contrast (272 mL vs. 302 mL, p < 0.001) and lower procedural cost ($5,332 vs. $6,007, p < 0.001).
The exact mechanism of benefit in the FFR-guided PCI arm of the FAME trial is not known; however, it appears to support the well-recognized fact that the most important prognostic indicator for adverse outcome in patients with CAD is the presence and extent of ischemia.10 It is possible that FFR-guided PCI results in a net clinical benefit as the reduction in ischemia by PCI outweighs the risks of PCI (stent thrombosis, restenosis) due to fewer implanted stents. The beneficial effects of FFR-guided PCI were maintained out to 2 years on follow-up in the FAME study.11 The purpose of the randomized FAME II trial is to compare outcomes of FFR-guided PCI plus OMT versus OMT alone in patients with stable CAD.
CORONARY INTRAVASCULAR IMAGING
Coronary angiography, which provides a luminogram, does not provide information about the atherosclerotic plaque in the vessel wall and often underestimates the severity and extent of atherosclerosis. Over the last two decades, IVUS has evolved as adjunct to coronary angiography and provides valuable information about the vessel wall with several research and clinical applications. Recently, infrared light-based imaging technology such as OCT has emerged, which provides significantly improved image resolution compared to IVUS.
Intravascular Ultrasound
The IVUS equipment consists of a special transducer mounted catheter and a console to reconstruct and display the image. Current IVUS catheters range from 2.6 to 3.5 French (Fr) in size and can be advanced through a conventional 6-Fr guide catheter over a 0.014-inch angioplasty guidewire. There are two types of IVUS catheters, phased array and rotating transducer. High ultrasound frequencies (20 to 40 MHz) are employed resulting in excellent axial and lateral resolution. Intravenous heparin and intracoronary nitroglycerin are routinely administered before performing IVUS. Subsequently, the angiographer retracts the transducer manually or with a motorized pullback device. Images are obtained and recorded digitally for analysis during pullback, using side branches visualized with both angiography and ultrasound, as landmarks to facilitate interpretation. IVUS is a safe procedure with few documented complications. Coronary spasm is the most frequent complication (1% to 3%) and this responds well to intracoronary nitroglycerin. Major complications including dissection or vessel occlusion are rare (<0.5%).
Normal Coronary Anatomy by IVUS
In normal coronaries, a standard IVUS image usually shows the vessel wall as a trilaminar structure, consisting of the intima, media, and adventitia (Fig. 46.2), due to visualization of two strong acoustic interfaces by ultrasound, the leading edge of the intima (at the interface between the blood-filled lumen and the endothelium) and the external elastic membrane (EEM, located at the media–adventitia interface). The lumen shows swirling echoes from circulating blood elements and this “blood speckle” helps identify dissection planes.
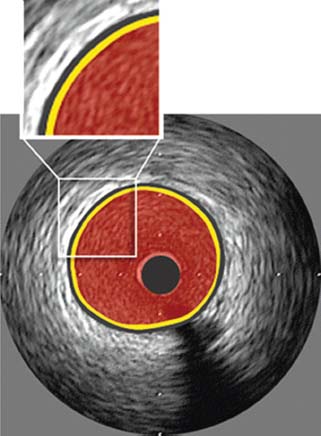
FIGURE 46.2 Normal coronary artery anatomy by IVUS. Yellow color represents intima; black color represents media; red color represents blood-filled lumen. (Reproduced from Tuzcu EM, Bayturan O, Kapadia S. Invasive imaging: Coronary intravascular ultrasound: a closer view. Heart. 2010;96:1318–1324, with permission from BMJ Publishing Group Ltd.)
Characterization of Atherosclerosis by IVUS
IVUS studies have shown that atherosclerosis is much more extensive and severe than what is apparent on coronary angiography. Atherosclerotic plaques can range in appearance from soft or hypoechoic plaques that have high lipid content to bright echogenic plaques that have more fibrous and calcified components. A more detailed analysis of plaque composition is possible using IVUS-derived virtual histology (IVUS-VH), which has good histopathologic validation and is based on spectral analysis of radiofrequency signals. Using IVUS-VH, atherosclerotic plaque is color coded into different types based on lipid, fibrous, and calcium content. Studies have shown a greater proportion of lipid and necrotic core with a thin fibrous cap in patients presenting with acute coronary syndrome.12 Another interesting concept revealed by IVUS studies is arterial remodeling (positive or negative), which refers to changes in arterial dimensions associated with the development of atherosclerosis. Positive or expansive remodeling refers to increase in the lumen area in the initial stages of atherosclerosis due to expansion of the EEM area with plaque deposition.13 This may explain the discrepancy between IVUS findings of significant plaque and normal or mildly abnormal coronary “luminogram” by angiography. It has been shown that lesions associated with expansive remodeling are prone to rupture and lead to acute coronary syndromes.14 IVUS studies have also demonstrated the other kind of remodeling, referred to as negative remodeling or arterial shrinkage, which is more common in stable CAD.15 It has been implicated in restenosis following balloon angioplasty. Precise quantitation of the extent of atheroma at different time points using IVUS has been instrumental in understanding the natural history of atherosclerosis as well as the impact of cholesterol-lowering drugs in reducing the progression of atherosclerosis16 and even its regression.17
 The first large prospective, randomized multicenter IVUS trial of statins, the REVERSAL (Reversal of Atherosclerosis with Aggressive Lipid Lowering) study, randomly assigned patients to receive a moderate lipid-lowering regimen consisting of 40 mg of pravastatin or an intensive lipid-lowering regimen consisting of 80 mg of atorvastatin.16 With significantly greater reductions in LDL and CRP levels, the intensive lipid-lowering regimen halted the progression of coronary atherosclerosis as assessed by IVUS, compared to the moderate lipid-lowering regimen.
The first large prospective, randomized multicenter IVUS trial of statins, the REVERSAL (Reversal of Atherosclerosis with Aggressive Lipid Lowering) study, randomly assigned patients to receive a moderate lipid-lowering regimen consisting of 40 mg of pravastatin or an intensive lipid-lowering regimen consisting of 80 mg of atorvastatin.16 With significantly greater reductions in LDL and CRP levels, the intensive lipid-lowering regimen halted the progression of coronary atherosclerosis as assessed by IVUS, compared to the moderate lipid-lowering regimen.
 The prospective ASTEROID study (A Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden) evaluated coronary atherosclerosis by IVUS before and after 24 months of intensive lipid-lowering therapy with 40 mg/d of rosuvastatin.17 With a lowering in mean LDL-C to 61 mg/dL and an elevation in HDL-C by 14.7%, significant reduction in atheroma burden was observed by IVUS, consistent with regression of coronary atherosclerotic plaque.
The prospective ASTEROID study (A Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden) evaluated coronary atherosclerosis by IVUS before and after 24 months of intensive lipid-lowering therapy with 40 mg/d of rosuvastatin.17 With a lowering in mean LDL-C to 61 mg/dL and an elevation in HDL-C by 14.7%, significant reduction in atheroma burden was observed by IVUS, consistent with regression of coronary atherosclerotic plaque.
Applications of IVUS in the Catheterization Laboratory
It is common to encounter lesions on angiography that are of uncertain significance such as angiographically moderate stenosis (50% to 70%), difficult to assess sites such as ostial or bifurcation lesions, left main trunk disease, intraluminal filling defects, and hazy lesions. Unlike FFR, specific threshold criteria for intervention by IVUS have not been prospectively validated.
 A minimal luminal area of 3 to 4 mm2 by IVUS correlates with a significant reduction in FFR for a native coronary artery18 and a minimal luminal area of <6 to 7.5 mm2 usually indicates a significant lesion in the left main trunk.19,20
A minimal luminal area of 3 to 4 mm2 by IVUS correlates with a significant reduction in FFR for a native coronary artery18 and a minimal luminal area of <6 to 7.5 mm2 usually indicates a significant lesion in the left main trunk.19,20
Cardiac allograft vasculopathy is known to be associated with a poor outcome in heart transplant patients. IVUS allows assessment of early plaque accumulation before luminal stenosis develops, thus recognizing silent vasculopathy, which has been identified as a powerful predictor of subsequent adverse outcomes in transplant recipients.21 IVUS has several important applications before, during, and after PCI.
 Pre-PCI IVUS allows assessment of plaque distribution, especially whether the lesion involves or spares the ostium of the vessel that may help with optimal balloon and stent positioning. The presence and distribution of calcification can be assessed with IVUS.
Pre-PCI IVUS allows assessment of plaque distribution, especially whether the lesion involves or spares the ostium of the vessel that may help with optimal balloon and stent positioning. The presence and distribution of calcification can be assessed with IVUS.
 Stent undersizing is a well-known predictor of stent thrombosis,22 and IVUS can help with selecting the optimal stent size based on vessel and lumen size.
Stent undersizing is a well-known predictor of stent thrombosis,22 and IVUS can help with selecting the optimal stent size based on vessel and lumen size.



