Chapter 11
Catheter Malfunction: Diagnosis and Treatment
The widespread use of long-term central venous access devices has been emphasized throughout this book. Proficiency in the placement of these catheters is important because the catheter will function well only if it is placed well. Catheter care is also important because even a well-placed catheter is of little use if it becomes infected and must be removed. Equally important are the follow-up and maintenance of malfunctioning catheters because most central venous catheters eventually will malfunction.
Multiple noninfectious complications can threaten catheter longevity. Fibrin sheath formation at the catheter tip is ubiquitous. The effects of fibrin sheath or thrombus formation at the catheter tip can range from the inconvenience of persistent withdrawal occlusion in portacaths and non-dialysis tunneled catheters to rendering catheters clinically useless, especially if high flow rates are required, such as in dialysis. Major efforts of catheter maintenance are required to maintain flow rates adequate for hemodialysis, that is, greater than 250 to 300 mL per minute. Silastic cuffed hemodialysis catheters have been used increasingly for providing long-term vascular access and now account for 10 to 15% of access in most dialysis facilities.1 Dialysis catheter malfunction is common, occurring in 87% of catheters before they were removed in a study by Suhocki and colleagues.2 The mean time from catheter insertion to the first malfunction was 2.8 months. In another study, the primary patency of hemodialysis catheters at 3 and 6 months was 54% and 33%, respectively, with a median time to initial failure of 3.5 months.3
Historically, catheter failure was managed by placement of a new catheter via a new access site.4 The use of other central veins for access can compromise future access sites, however, and access sites quickly become depleted. Repeated placement of hemodialysis catheters at different sites carries several risks, including the inherent risk of placing a new catheter and venous stenoses or occlusions. Cimochowski and co-workers5 showed marked venous stenoses in 50% of patients who previously had temporary subclavian vein or internal jugular vein dialysis catheters; mean catheter dwell time was just 11.5 days. Subclavian vein or innominate vein stenosis or occlusion threatens the outflow of an existing ipsilateral access graft or arteriovenous fistula or precludes its surgical placement. As the life expectancy of patients with chronic renal failure increases, preservation of existing central venous catheter sites becomes of paramount importance.6
HISTORY AND EXAMINATION
A “simple catheter check” can provoke nonchalance and disinterest of vascular and interventional radiologists even to the extreme that the study is done by a nonphysician member of the venous access team and may be checked by the radiologist afterwards. This situation should be discouraged. The vital importance of the access to the patient must be kept in mind, and everything possible must be done to keep these catheters functional. The vascular and interventional radiologist should be involved from the start. The history and examination can give valuable clues for making a correct diagnosis. Certain symptoms or signs can point to the underlying problem with the catheter.
Pain
Pain during infusion suggests an extravasation of the infused solution into the soft tissues. The location of the pain is a guide as to where the problem lies. There are several causes of extravasation from a venous access device:
• The portacath is not adequately accessed with the access needle.
• The catheter has become disconnected from the port.
• The catheter is fractured (Fig. 11–1).
• The catheter is poorly positioned and has migrated out of the vein.
• Venous thrombosis has developed.
• Fibrin sheath has formed around the catheter.
Inability to Aspirate
Persistent withdrawal occlusion (PWO) is a common presentation of a catheter with fibrin sheath or thrombus at the tip of the catheter acting as a one-way valve. This usually occurs several weeks after placement. If PWO occurs immediately after catheter placement, the problem is most likely due to the catheter being malpositioned with the catheter tip against the wall of the vein. In non-high-flow catheters, PWO can prove to be inconvenient by preventing blood aspiration and yet allowing infusions. In high-flow catheters, however, PWO can quickly lead to complete loss of catheter function because of the inability to sustain necessary high flow rates.

Figure 11–1 (A) High puncture has resulted in kinking of the catheter. (B) The kinked catheter has broken, causing extravasation into the soft tissues. There is also leakage from the port, possibly due to high resistance to injection (due to the kink) causing leakage around the needle.
Poor Flow Rates
When poor flow rates occur shortly after catheter placement, they are usually due to improper tip position, subcutaneous kinking of the catheter, or extrinsic compression of the catheter owing to tight fixation device or sutures around the catheter, constricting the lumen. Catheters that are too short or too long or kinked in the subcutaneous tunnel will not function well and will require correction or replacement. Misplacement of catheters is more likely to occur when inserted without imaging guidance. The rate of catheter malposition is as high as 29% on postprocedure radiographs when fluoroscopy is not used for placement.7 With the use of fluoroscopic guidance, primary misplacement occurs in fewer than 2% of cases.2
Swelling
Swelling of an extremity, both extremities, or the head and neck may indicate venous thrombosis and possibly the superior vena cava (SVC) syndrome. Venography or sonography is needed to evaluate venous thrombosis. Often, the indwelling catheter will function adequately in such circumstances because the thrombus is present at the catheter insertion site or along the intravenous portion of a catheter and not necessarily at the catheter tip. Likewise, a catheter check—injecting contrast through the indwelling catheter and obtaining radiographs—may be perfectly normal even in the face of widespread venous thrombosis. Venous thrombosis is discussed in Chapter 13.
TECHNIQUE FOR CHECKING CATHETERS
Before performing digital subtraction imaging, the catheter dressing should be removed and the skin site and external portion of the catheter examined. The dressing should be checked for drainage. Examining the skin for signs of infection, such as erythema and tenderness, is an important step if catheter exchange over a guidewire is being considered, because a new catheter should not be placed in an infected tunnel or exit site. It is also important to examine the portion of the catheter that is used for clamping the catheter. Occasionally, the stiff plastic may become kinked as a result of repeated clamping, limiting the flow. One must ensure that a suture or fixation device has not been placed too tightly around the external portion of the catheter, compressing the lumen and restricting flow.
The catheter hub should be prepared with Betadine and laid on sterile towels. The indwelling heparin must be discarded. The ability to aspirate is an important finding and should be reported. As previously discussed, the ability to inject but not aspirate through a catheter indicates a one-way valve-like mechanism, usually due to one of three causes: (1) fibrin sheath at the catheter tip, (2) thrombus at the catheter tip, or (3) catheter tip against the wall of the vein. Removal of the heparin is very important. Typically, dialysis catheters are blocked with 5000 U of heparin in each lumen. Injection of 10,000 U of heparin into the bloodstream can cause systemic heparinization, making removal of the catheter and placement of a new catheter dangerous.
HELPFUL HINT
If one port will not aspirate and heparin cannot be removed, avoid injecting contrast through this lumen and use the other lumen to check the catheter. In most instances, injection of a single lumen will provide adequate information. Even if the injection through the one lumen is not diagnostic, it can be assumed that a problem is present, and correction is done based on this information.
The entire length of the catheter must be checked fluoroscopically. Occasionally, constriction of the catheter by a tight suture or fixation device will be evident by fluoroscopy even if it is not noticed by visual inspection. Also, a mechanical kink in the catheter, which is most often (but not always) present at the site of transition from the subcutaneous tunnel into the vein, may be found. Occasionally, when the jugular vein has been used for venous access, a mechanical kink becomes evident only in a particular head position. Therefore, the venous entry site is examined fluoroscopically while the patient rotates his or her head. The position of the tip of the catheter should also be evaluated with fluoroscopy. Contrast should be injected while obtaining digital images (Fig. 11–2). The examination should be tailored to the suspected problem, based on the history. Specifically, if the history indicates extravasation into the soft tissues, a small amount of contrast should Figure 11–2 Catheter check technique of injecting contrast while obtaining images at a rapid rate. Fibrin sheath and thrombus can be seen better if the image is magnified and collimated over the catheter tip. be slowly injected under fluoroscopy or digital imaging of the entire length of the catheter. Only a small amount of contrast is needed to visualize leakage into the soft tissues from a rupture of the catheter or to visualize a retrograde “backtracking” along the outer wall of the catheter caused by fibrin sheath or thrombus around the catheter. On the other hand, if the clinical history indicates PWO, a larger injection of contrast is necessary and imaging should focus on the catheter tip. Using a 20-mL syringe filled with contrast, injection should be done as rapidly as possible with images of the catheter tip obtained at a rapid filming rate of two or three frames per second. Use of the 20-mL syringe should eliminate the risk of catheter rupture because of the low-pressure injection generated by this syringe. The contrast should be flushed from the catheter as soon as possible and the catheter blocked with heparin to avoid thrombosis.
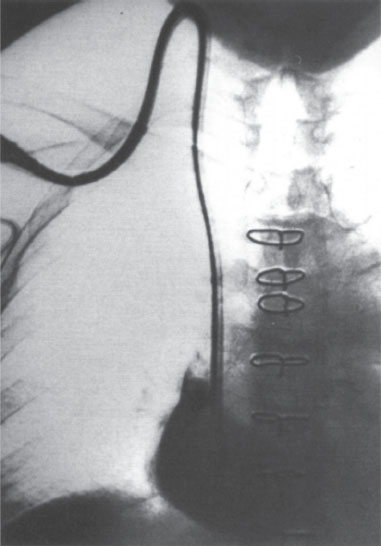
Figure 11–2 Catheter check technique of injecting contrast while obtaining images at a rapid rate. Fibrin sheath and thrombus can be seen better if the image is magnified and collimated over the catheter tip.
HELPFUL HINT
In patients with a history of allergic reaction to iodinated contrast or in patients with renal insufficiency but who are not on dialysis, gadolinium with digital subtraction imaging may be used as an alternative to iodinated contrast.
It is our opinion that a catheter injection with imaging should be performed every time a catheter exchange is performed. Venous thrombosis must be diagnosed if a new catheter is to be placed. It is especially important to check catheters placed by other services before exchange is performed to ensure adequate intravenous location of the catheter to avoid serious complications (Fig. 11–3).
CATHETER CHECK: FINDINGS OF PERSISTENT WITHDRAWAL OCCLUSION
The angiographic signs of fibrin sheath at the catheter tip are variable and are often difficult to interpret. The “classic” well-described signs include the following:
• Filling defects associated with either port of the catheter (Fig. 11–4)
• Reflux of contrast material along the proximal shaft of the catheter with efflux from defects in the fibrin sleeve (Fig. 11–5)
• Excessive ejection of contrast from side holes when injecting the proximal port
• Lack of contrast material jet flowing into the right atrium (RA) (Fig. 11–6)
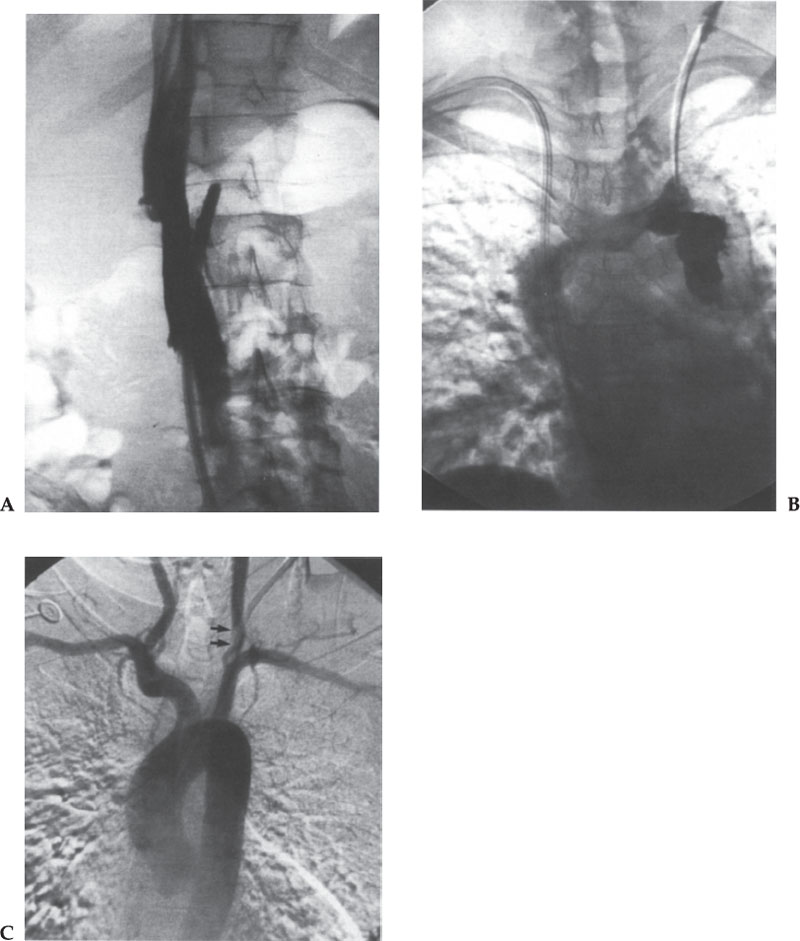
Figure 11–3 Several examples of why a catheter check should be performed before catheter exchange over a guidewire is done. (A) The inferior vena cava (IVC) catheter was found to be outside of the IVC, either through the IVC wall or in a small side branch of the IVC. (B) Contrast injection of a surgically placed malfunctioning catheter revealed extravasation caused by the extraluminal position of the catheter tip. (C) A permacath placed at another hospital had malfunctioned and bled for more than a month, and we were asked to exchange the catheter. Contrast injection, however, showed the catheter to be intra-arterial. Thrombus was present at the entrance site in the left common carotid artery (arrows). The vascular surgeons removed the catheter by a cut-down to protect the carotid artery distribution, and the common carotid artery was thrombectomized. If this catheter had been exchanged over a wire, the thrombus would likely have embolized to the brain.
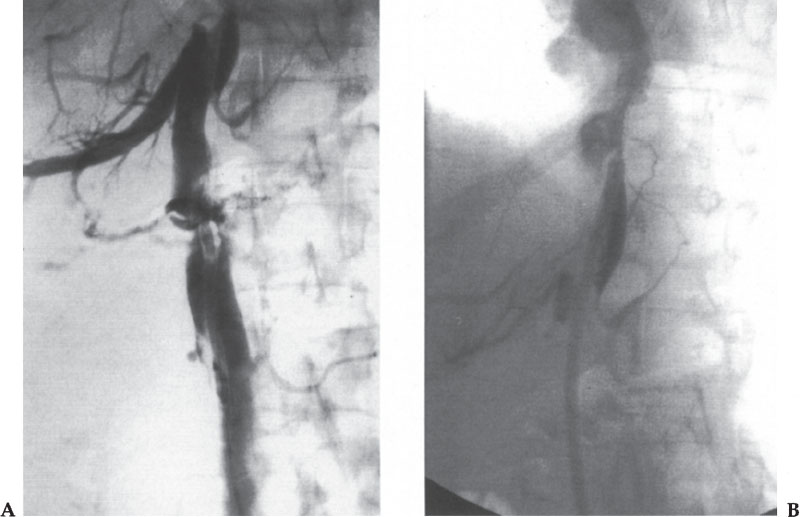
Figure 11–4 (A, B) Appearance of fibrin sheath. Filling defects are seen surrounding the catheter tips. A “halo” can be appreciated around the catheter.
It is quite possible, however, that the angiogram done to check the catheter may not show any abnormality. As stated, interpretation of the study is often difficult because of motion of the catheter caused by cardiac motion and because the sleeve of fibrin can be thin and difficult to see. If no problems are found (kinking, malposition, thrombus, or obvious fibrin sheath) in a patient with PWO, fibrin sheath at the tip can be assumed to be the cause of catheter malfunction and should be treated accordingly.
The findings of thrombus formation at the catheter tip depend on the amount of the thrombus. If nonocclusive thrombus is present, a filling defect or defects should be evident on the angiogram, adhering to the catheter or the wall of the vein (Fig. 11–7). At the other end of the spectrum, the entire SVC may be thrombosed. In this case, one often sees retrograde flow into the azygous arch and azygous vein draining to below the diaphragm, into the inferior vena cava (IVC) (Fig. 11–8).
TREATMENT
Early Catheter Malfunction
Catheters must be inserted correctly to function satisfactorily. Catheter malfunction within the first several days of placement is usually due to improper catheter tip position, subcutaneous kinking, or extrinsic compression resulting from excessive tightening of sutures or fixation devices around the catheter. Finally, a new catheter placed into existing thrombus or fibrin sheath, which is a residual from a previously placed catheter, will malfunction early.
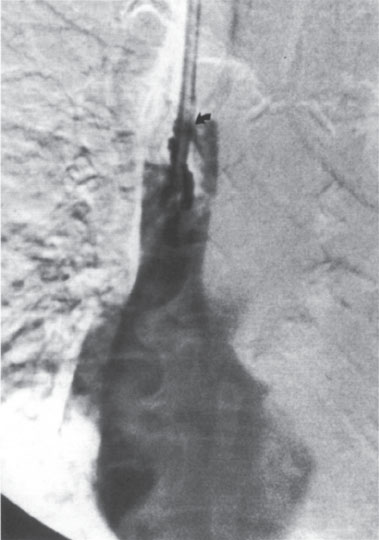
Figure 11–5 Appearance of fibrin sheath. Contrast refluxes along the catheter, within the fibrin sheath, until it effluxes through a defect in the sheath (arrow).
Suture Constriction
Sutures or fixation devices that impinge on the lumen of the catheter should be removed and a new suture or fixation device placed. Great care must be taken when removing the sutures to avoid cutting the catheter. Removal is done as follows:
1. With an assistant applying counter-resistance to the catheter, firmly grasp and pull the knot with pick-ups or a clamp.
2. Use a scalpel or scissors to cut the knot as close to the catheter as possible without cutting the catheter. One need not cut against the catheter because Figure 11–6 Appearance of fibrin sheath. The contrast fills a fibrin sheath extending from the tip of the catheter, causing a “wind sock” appearance. suture material with “memory” will unravel when the knot is cut. For example, if the knot has been tied with three or four throws, attempt to cut between the first and second throws (closest to the catheter), which should allow unraveling of the final throw. This process may need to be repeated if more than one knot was tied.
3. Place a new suture or fixation device.
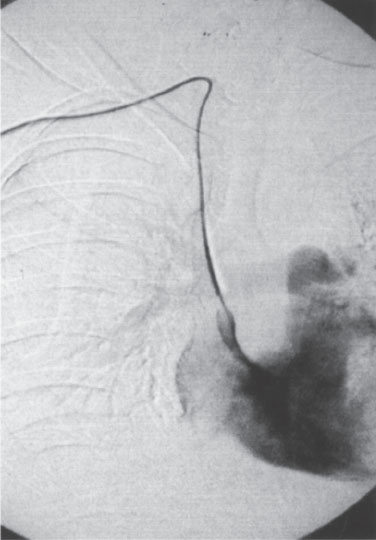
Figure 11–6 Appearance of fibrin sheath. The contrast fills a fibrin sheath extending from the tip of the catheter, causing a “wind sock” appearance.
Catheter Kinking
Catheter kinking is frequently the result of a venous puncture too high in the neck or the result of improper subcutaneous tunneling, which results in too acute of an angle at the venous entry site (Fig. 11–9). Placing a guidewire can straighten the kink for temporary relief only; the kink often will reappear when the guidewire is removed because the underlying problem (poor tunneling or poor access site) has not been corrected. Likewise, catheter exchange over a guidewire is unlikely to solve the problem because, even though a new catheter has been placed, the underlying problem of poor tunnel angulation is still present. The best solution is either placement of a new catheter de novo or retunneling using the same venous puncture site (Fig. 11–10). The retunneling procedure is done as follows:
1. After sterile preparation, use a no. 10 or no. 15 scalpel to reopen the incision at the venous entry site.
2. Use a clamp to retrieve the catheter at the incision.
3. Clamp the catheter and cut on the subcutaneous tunnel side of the catheter (i.e., away from the vein).
4. Advance a guidewire through the venous segment of the catheter, which is removed over the guidewire.
5. Insert a peel-away sheath of the appropriate size over the guidewire.
6. Remove the remaining catheter from the subcutaneous tunnel.
7. Create a new subcutaneous tunnel so that the angulation at the venous entry site is less acute.
8. Then insert the catheter in the usual fashion.
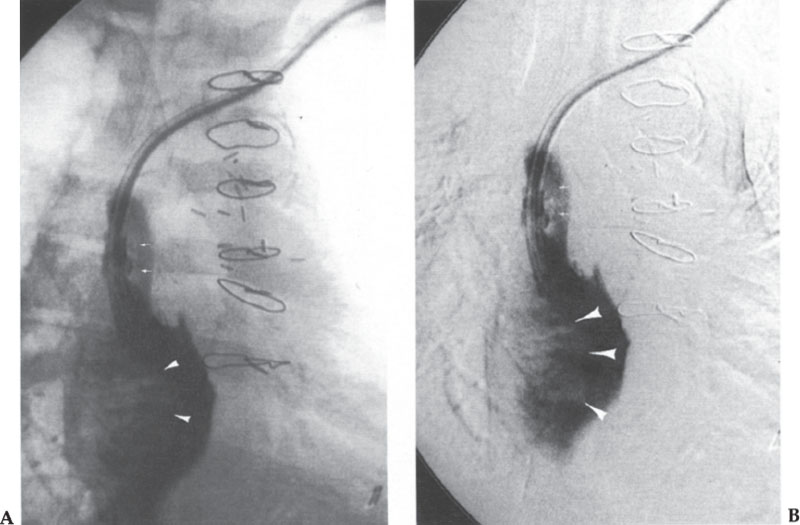
Figure 11–7 (A, B) Fibrin sheath and thrombus are seen as filling defects along the catheter (small arrows) with a large thrombus extending into the right atrium (arrowheads).
Placement of a new catheter can be performed at a different access site or in the same vein. The latter technique is useful when the original venous puncture was too high in the neck, causing catheter kinking and malfunction. Replacement of the catheter via the same venous puncture site may not resolve the problem associated with the catheter, and it is usually better to perform a new puncture, lower in the neck, under sonographic guidance or under fluoroscopic guidance using the indwelling catheter as a landmark (Fig. 11–11). This can be performed even if the vein is thrombosed.
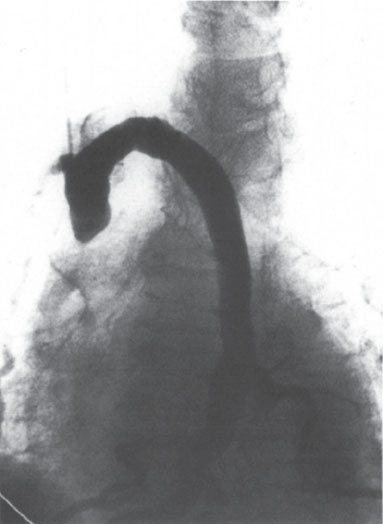
Figure 11–8 There is complete thrombosis of the superior vena cava (SVC) with collateral flow into the azygous arch.



