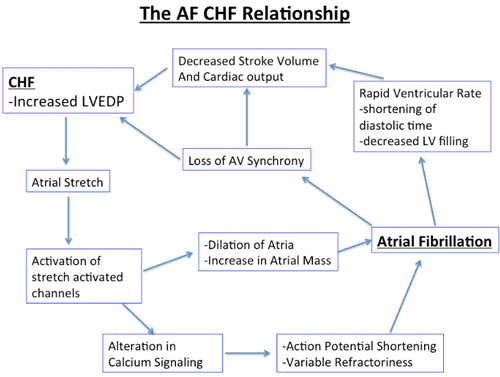
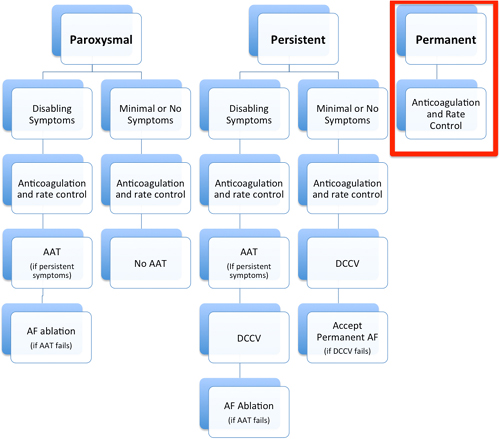
CHAPTER 17 Yousef Bader, MD, and Diane Grogan, NP A 73-year-old gentleman with a history of hypertension, chronic obstructive pulmonary disease, lung cancer, which was in remission after surgical resection and radiation therapy, coronary artery disease (CAD) with an ischemic cardiomyopathy and a left ventricular ejection fraction (LVEF) of 15% was referred to our institution for evaluation of permanent atrial fibrillation. The patient had a dual-chamber biventricular (BiV) implantable cardioverter-defibrillator (ICD) placed for cardiac resynchronization therapy (CRT), but over a period of a few months, developed worsening heart failure, New York Heart Association (NYHA) class III symptoms in the setting of permanent atrial fibrillation (AF) with a ventricular rate in the 120s. His blood pressure was 90/47 mm Hg, which was consistent with prior visits. Interrogation of his device revealed true BiV pacing to be less than 30% of the time due to his AF as evidenced by his electrocardiogram (ECG) (Figure 17.1). The patient’s cardiac medications were metoprolol XL 25 mg daily, digoxin 0.125 mg daily, furosemide 20 mg daily, and warfarin 2.5 mg daily. Rhythm-control strategies had previously failed and rate-control strategies were limited due to a baseline low blood pressure. In this chapter, we review the pathophysiology, and management strategies of patients suffering from both permanent AF and congestive heart failure (CHF). ECG of the patient from our case presentation. Underlying rhythm is AF with a left bundle branch block (LBBB). The American College of Cardiology, American Heart Association, and the European Society of Cardiology developed guidelines for the classification and management of patients with AF. AF is classified as paroxysmal, persistent, longstanding persistent or permanent. Paroxysmal AF is defined as episodes that come and go, but resolve spontaneously within 7 days. By contrast, AF is considered persistent when episodes last longer than 7 days or require chemical or electrical cardioversion. AF is considered longstanding persistent when it is continuous and lasts longer than one year. Finally, permanent AF is defined as persistent AF in which a decision has been made not to try to restore normal sinus rhythm (SR). Because in permanent AF rhythm control is not desired, goals of therapy are limited to controlling ventricular rates, anticoagulation to prevent strokes and alleviating patient symptoms (Figure 17.2).1 Management of paroxysmal, persistent, and permanent AF. Source: Modified from January CT, Wann LS, Alpert JS, et al., J Am Coll Cardiol. 2014;64(21):e1–e76. “AF begets AF” is a teaching that was first introduced by Dr. Wijffels et al.2 It is clear that the longer patients are in AF, the less likely a rhythm-control strategy will be successful. Electrical and structural remodeling of the atria leads to stabilization of AF and eventually results in permanent AF, which is defined as longstanding persistent AF, in which a rhythm-control strategy is no longer pursued. AF in patients with CHF poses particular concern because of the symbiotic relationship between these conditions. Based on the Framingham Heart Study, approximately one-third of patients presenting for the first time with CHF have concomitant AF and vice versa.3 There is also a direct relationship between severity of CHF and prevalence of AF.4 It is not surprising that these two conditions coexist as they share many common predisposing risk factors including hypertension, CAD, obesity, and genetic conditions involving SCN5A mutations. The effects of CHF on AF are, in part, a result of atrial stretch, which occurs to accommodate increases in left ventricular end diastolic pressure. Atrial stretch influences stretch-activated channels, which leads to alterations in calcium signaling, action potential shortening, and variable refractoriness. As the atria continue to dilate, increase in atrial mass further contributes to initiation and maintenance of AF.5,6 AF can also contribute to worsening heart failure by two main mechanisms. Since ventricular rates are faster in AF, this causes a shortening of diastolic time and LV filling, resulting in a decreased stroke volume and cardiac output. Prolonged periods of AF with rapid rates can result in a tachymyopathy. In addition, loss of atrioventricular (AV) synchrony and the atrial kick result in added compromise to LV diastolic function7 (Figure 17.3). Mechanisms of CHF and AF. The management of patients with heart failure and reduced ejection fraction (HFREF) has dramatically improved over the last two decades with increased survival and more optimal symptom management. The management of AF has also seen advances with the development of ablative procedures and novel anticoagulants to prevent stroke. The management of patients with both permanent AF and HFREF has remained a challenge. Many randomized controlled trials have compared rate control to rhythm control and found no significant difference between the two, including the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) and the Strategies for Treatment of Atrial Fibrillation (STAF) trials.8,9 The Rhythm Control versus Rate Control for Atrial Fibrillation and Heart Failure (AF-CHF) trial randomized 1376 patients with CHF and AF to rhythm versus rate control and found no difference in death, symptoms, functional status, or LV function.10 On the basis of post hoc analysis of the US Carvedilol Heart Failure trial, we know that β-blocker use in patients with CHF and AF leads to improved outcomes with regards to death and heart failure hospitalizations. Whether this is due to rate control or other benefits of β-blockers is unclear.11 The Rate Control Efficacy in Permanent Atrial Fibrillation: a Comparison Between Lenient versus Strict Rate Control (RACE II) study found no statistically significant difference between lenient and strict rate-control strategies.12 Some cardiologists are critical of the RACE II trial because the difference in heart rates between the two groups was not dramatic (93 ± 9 vs. 73 ± 12). Although there were a small number of patients with heart failure in the RACE II trial, a subgroup evaluation of that group of patients also found no difference in lenient versus strict rate control (Figure 17.4).13 Summary of the RACE II trial. β-Blockers: According to the HF guidelines, using β-blockers for rate control in patients with chronic heart failure is a class IA indication.14 This is supported by several studies demonstrating the safety and efficacy of these agents, with decreased morbidity and mortality with their use specifically in the heart failure population. Metoprolol XL and carvedilol have been shown to have a mortality benefit in patients with a reduced ejection fraction, so should be first-line in this population.15 Digoxin: In certain patients, digoxin can be used alone or in conjunction with β-blockers for ventricular rate control. Digoxin has been shown to decrease heart failure hospitalizations in patients with CHF but has no effect on mortality.16 Follow up of patients who have been taking digoxin has shown that although rate control at rest is easily achieved, digoxin fails to control heart rates during exercise. This supports the concurrent use of β-blockers.17 Digoxin should be used with caution due to its narrow therapeutic index. This is particularly true for patients with renal dysfunction and hypokalemia. The adverse effects of digoxin include bidirectional ventricular arrhythmias, AV block, and atrial tachyarrhythmias.18 Nondihydropyridine calcium channel blockers: Nondihydropyridine calcium channel blockers including verapamil and diltiazem are highly effective at controlling ventricular rates in patients with AF. Despite this, in patients with systolic heart failure, their use should be avoided because of their negative inotropic effects.19 Amiodarone: The use of amiodarone in CHF patients should be second-line, reserved for those who fail or have contraindications to conventional therapy with β-blockers and digoxin.20 Although it has been shown to be safer than digoxin, its long-term use is associated with pulmonary, thyroid, and hepatic dysfunction among other adverse effects. Amiodarone is highly effective at controlling ventricular rates and is particularly useful in the critically ill patient (Table 17.1).21,22
Case Study: Permanent Atrial Fibrillation
CASE PRESENTATION

AF DEFINITIONS, TREATMENT GOALS, AND NATURAL HISTORY
MECHANISMS/PATHOPHYSIOLOGY OF AF IN HEART FAILURE
MANAGEMENT OF AF IN CHF PATIENTS
VENTRICULAR RATE CONTROL
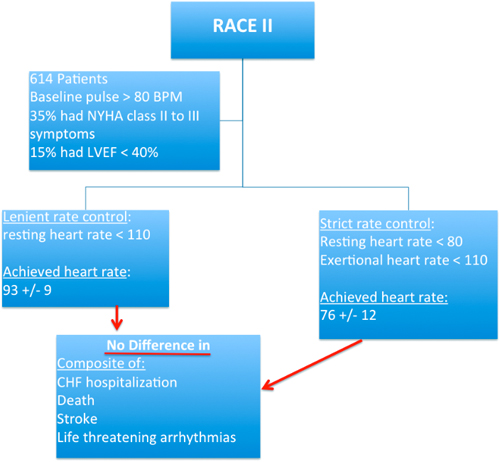
Table 17.1 | |
Group | Recommendations |
European Society of Cardiology (2010) | Start with lenient rate control (resting heart rate less than 110 bpm). Strict rate control (resting heart rate less than 80 bpm or with exercise less than 110 bpm) should be reserved for those with a tachymyopathy or persistent symptoms. |
American College of Cardiology ACC and American Heart Association AHA and Heart Rhythm Society | Strict rate control (resting heart rate 60–80 bpm and with exercise 90–115 bpm) |
Chronic HF Guidelines | Recommend a lenient rate control (resting heart rate less than 80–90 bpm and less than 110–130 bpm with exercise) |
UPSTREAM THERAPY AND PREVENTION OF AF
It is important to recognize that patients with CHF are at a higher risk of AF because upstream therapy may be useful in this patient population. Some studies have suggested that renin–angiotensin–aldosterone inhibition has a role in prevention of AF. In an animal study, it has been shown that enalapril reduced the effects of CHF on atrial conduction and atrial fibrosis.23 In humans, analysis from the TRACE study and the SOLVD trials suggest that angiotensin converting enzyme inhibitors can reduce the occurrence of AF in patients with LV dysfunction.24,25
CRT AND AF
It is clear that patients in SR with CHF who meet indications for CRT have gained significant mortality and symptomatic benefit from this therapy. However, the percent biventricular pacing is critically important. Benefit with symptom improvement and ventricular remodeling is actualized only in those patients who have greater than 95% ventricular pacing. The US LATITUDE trial was the largest study demonstrating that the higher the percent of BiV pacing, the better the outcomes. The authors reported that greater than 98% pacing was associated with significant reductions in mortality. Patients with 99.6% BiV pacing had a 24% reduction in mortality compared with a 19% reduction in patients BiV pacing 94.8% of the time.26 Unfortunately, patients with AF who obtain CRT therapy have not had the same benefits. A number of reasons contribute to this and this was shown in a several studies. A sub-analysis of the MADIT-CRT showed that patients with CRT and AF had increased mortality and heart failure events.27 Boriani et al. demonstrated that patients in AF had 71% pacing compared to 98% in SR.28 The percent pacing in AF can also be misleading because the device counters do not account for fusion and pseudo-fusion, which are not effective BiV paced beats and do not contribute to the benefits of CRT. This was demonstrated by Kamath et al., who evaluated Holter monitors of patients with CRT and AF. They found that although device interrogation recorded more than 90% pacing, less than 50% of patients truly had effective BiV pacing based on Holter tracings.29
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree