CHAPTER 15 Mohamed Aljaabari, MD, Angelo Francisquini, RN, and Lisa Contis, RN, NP A 76-year-old female with a past medical history of coronary artery disease (CAD), hypertension, hyperlipidemia, prior transient ischemic attack (TIA), and diabetes mellitus (DM) controlled by oral medication was initially admitted 2 days previously with pneumonia and was being treated with antibiotics. The patient developed new-onset atrial fibrillation (AF) with rapid ventricular rates (RVR) that was noted on telemetry without symptoms. Her medication regimen at the time was aspirin, atorvastatin, lisinopril, metoprolol succinate, metformin, and moxifloxacin. Her electrocardiogram (ECG) in AF is shown in Figure 15.1. Patient’s ECG in AF with RVR. This case presents several common clinical decision points that are encountered in patients with AF. These decisions revolve around 4 main goals in the management of acute AF: 1. deciding on the need for immediate cardioversion in the setting of acute AF, 2. control of the ventricular rate in AF, 3. making a choice between a rate- or rhythm-control strategy, and 4. evaluation of the patient’s thromboembolic and bleeding risk and making a risk-benefit assessment of the appropriate method for prevention of thromboembolism. The patient in this case has developed her first known episode of AF in an acute setting. When initially evaluating a patient with AF, the clinician must take a thorough history and perform a physical exam. It is important to determine how symptomatic the patient is with AF. If so, it is also important to document the duration, frequency, and initial onset of symptoms. This information is essential in guiding management decisions. As part of a thorough clinical evaluation it is also important to look for underlying predisposing conditions, such as valvular heart disease, thyroid disease, congestive heart failure (CHF), pulmonary disease, obstructive sleep apnea (OSA), and CAD. Treatment of any reversible condition is the initial step to prevent or reduce further episodes of AF. The social history should also focus on alcohol and caffeine consumption, as both are well-known triggers for AF. An ECG should always be obtained as well as other appropriate investigations, such as thyroid function tests and echocardiography, to screen for comorbid conditions.1 In this case, AF has occurred in the setting of an acute respiratory illness, which is not an unusual situation for AF. Conditions of physiologic stress such as sepsis, pulmonary embolism, respiratory illness, and the postoperative state may precipitate AF, especially in patients with predisposing factors such as advanced age or hypertension. The development of AF in these situations involves a complex interplay of physiological mechanisms including sympathetic stimulation, inflammatory mediators, myocardial fibrosis, and activation of the renin–angiotensin system.1,2 In these circumstances, AF will occasionally respond to treatment of the underlying condition and may resolve with its treatment. In other situations, the patient may have underlying AF that is discovered when presenting with another condition. Regardless of the presentation, AF needs to be managed with the aforementioned 4 main goals in mind. In certain circumstances, a patient may need urgent direct-current electrical cardioversion (DCCV), and a rapid assessment of the patient must be performed to make this decision. Immediate DCCV should only be considered when AF leads to hypotension with evidence of organ hypoperfusion, cardiac ischemia, or heart failure. The benefit of DCCV by reverting the patient to sinus rhythm (SR) should always be weighed against the potential risk of thromboembolism due to formation of left atrial (LA) thrombus. This risk increases significantly for episodes of AF lasting longer than 48 hours. In nonurgent situations, if the duration of AF is unknown or longer than 48 hours, then anticoagulation should be initiated and cardioversion delayed for 3 to 4 weeks with the patient on therapeutic anticoagulation.3 This strategy is well documented to reduce the risk of thromboembolism. Another option is to perform a transesophageal echocardiography (TEE) to rule out LA thrombus prior to cardioversion. This strategy was studied in the Assessment of Cardioversion Using Transesophageal Echocardiography Investigators (ACUTE) trial. In this study, 1222 patients with AF were randomized to 3 weeks of anticoagulation prior to DCCV versus TEE to rule out LA thrombus and a brief period of anticoagulation prior to DCCV. The results showed no significant difference in embolic events between the 2 groups.4 Anticoagulation should always be continued for at least 4 weeks after DCCV, as the immediate period after DCCV carries the highest risk of stroke.5 Cardioversion may also be achieved pharmacologically, but DCCV is more effective, faster, and obviates the need for monitoring for arrhythmias with QT-prolonging agents.1 It is important to consider that AF is rarely the sole culprit leading to a patient deteriorating hemodynamically, and the astute clinician must consider if addressing coexisting acute conditions may stabilize the patient and mitigate the need for immediate DCCV. Urgent cardioversion may fail, or the patient may revert to AF immediately after, if the underlying condition is not addressed. In these situations, initial rate control may be more desirable. In our case, the patient was asymptomatic with AF and hemodynamically stable, allowing for a more detailed assessment, as there was no urgent need for DCCV. The acute pneumonia was likely a trigger for AF in the setting of several comobidities including her age, hypertension, and CAD. Considering DCCV in her situation is still very reasonable in a first-episode of AF but should be deferred till the appropriate evaluation and treatment of underlying condition and embolic risk are addressed. Our patient was in AF with RVR with rates between 110 and 120 beats per minute (bpm). Another cornerstone of AF management is control of the ventricular rate in AF. Achieving rate control will improve patients’ symptoms and related morbidity.1 In some cases, sustained RVRs in AF may also lead to a cardiomyopathy that may be reversible after normalizing the rate or reverting to SR.6 The urgency for rate control depends on the how symptomatic the patient is and how rapid the rate is. In this situation, the patient was asymptomatic and the AF was in part related to acute illness. Therefore, tolerating some degree of tachycardia is expected as a physiological response to illness. Despite her severe tachycardia, it may be prudent to take a conservative approach to rate control. Aggressively lowering the heart rate may precipitate hypotension or bradycardia in response to accumulating doses of rate-controlling medications. The option of a more lenient rate-control goal in AF was studied in the Rate Control Efficacy in Permanent AF: a Comparison Between Lenient versus Strict Rate Control (RACE II) trial, which randomly assigned 614 patients with permanent AF to either a lenient rate-control strategy (resting HR < 110 bpm) or a strict rate-control strategy (resting HR < 80 bpm and HR during moderate exercise < 110 bpm) showing noninferiority of lenient rate control in the primary outcome of cardiovascular death, hospitalization for heart failure, and stroke, systemic embolism, bleeding, and life-threatening arrhythmic events.7 Several treatment options for rate control exist. The most effective and most widely used agents for rate control are agents that act to slow conduction over the AV node β-blockers (BB) and nondihydropyridine calcium channel blockers (CCB).8 Both of these have the limitation of causing hypotension and should be avoided in hypotensive patients. Furthermore, calcium channel blockers should be avoided in patients with reduced ejection fraction and BBs should be cautiously used in patients with severe obstructive lung disease.1 In patients where CCB or BB are contraindicated, one may use digoxin, a cardiac glycoside, which reduces the heart rate both through vagolytic effects and direct effect on AV nodal conduction. Digoxin dose does not cause hypotension but is not as efficacious, and due to its long half-life has a delayed effect to lower the heart rate, usually 6 hours following administration.9 As it is excreted renally, digoxin should be used with caution with dose adjustment in patients with chronic kidney disease. Furthermore, hypokalemia may potentiate digoxin toxicity and potassium levels should be monitored carefully, especially in the setting of diuretic use. All rate-controlling agents may be administered IV or orally depending on the severity of symptoms and rapid rates. Often gradual titration is needed to achieve adequate rate control, and combinations of rate-control drugs may be needed. It is important to avoid AV nodal blocking agents in patients with Wolff-Parkinson-White (WPW) syndrome as these agents may precipitate ventricular arrhythmia in AF by allowing conduction of rapid atrial rates to the ventricle through an accessory pathway.10 Amiodarone carries a class IIa indication for rate control of AF in the critically ill patient and can be utilized as a second-line agent for rate control. It is effective in controlling the heart rate when used in addition to or when other rate-control options are contraindicated. If amiodarone is prescribed for rate control, the clinician must be wary of the increased likelihood of cardioverting the patient chemically and the associated risk of thrombembolism. Similar to other IV rate-controlling agents, amiodarone given IV can cause hypotension and does have some AV nodal blocking effect. It should be also avoided in patients with WPW and AF with RVR.1 In our patient, a small dose of intravenous metroprolol was tolerated and showed an improvement in the heart rate. This was followed by doubling the oral BB dosage, which reduced ventricular rates in AF to between 100 and 110. Table 15.1 summarizes the available agents that may be used for rate control of AF and common formulations and dosages.
Case Study: Paroxysmal Atrial Fibrillation
CASE PRESENTATION
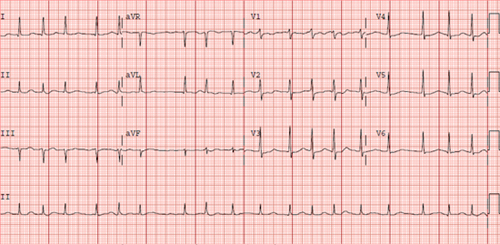
CASE DISCUSSION
Initial Evaluation
Deciding on the Need for Cardioversion
Rate Control
β-Blockers | Intravenous Dose | Oral Dose |
Metoprolol tartrate | 2.5–5.0 mg IV bolus over 2 min; up to 3 doses | 25–100 mg b.i.d. |
Metoprolol XL (succinate) | N/A | 50–400 mg q.d. |
Atenolol | N/A | 25–100 mg q.d. |
Esmolol | 500 mcg/kg IV bolus over 1 min, then 50–300 mcg/kg/min IV | N/A |
Propranolol | 1 mg IV over 1 min, up to 3 doses at 2 min intervals | 10–40 mg t.i.d. or 4x daily |
Nadolol | N/A | 10–240 mg q.d. |
Carvedilol | N/A | 3.125–25 mg b.i.d. |



