Case 21
HISTORY AND PHYSICAL EXAMINATION
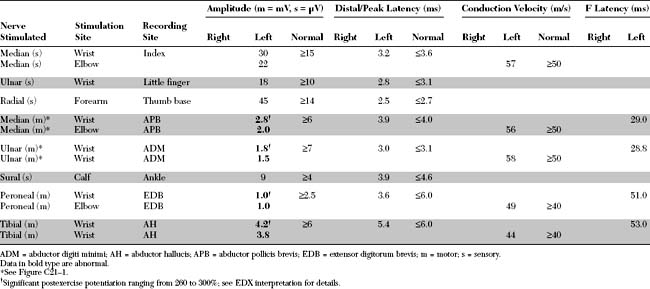
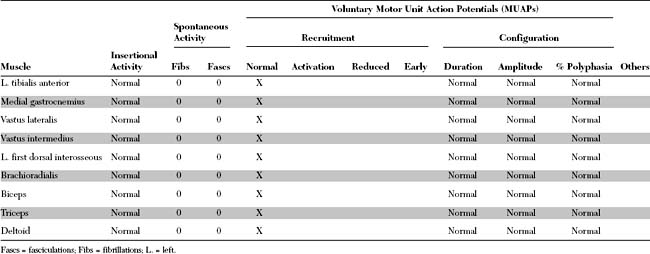
Please now review the Nerve Conduction Studies and Needle EMG tables.
QUESTIONS
EDX FINDINGS AND INTERPRETATION OF DATA
Relevant EDX findings in this case include:
DISCUSSION
Clinical Features
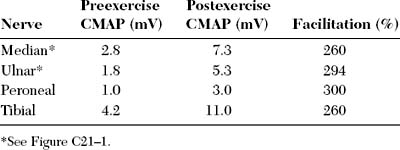
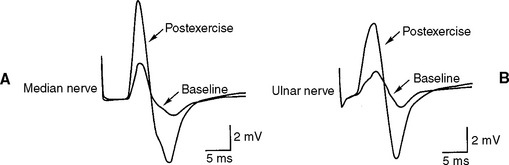
Lambert-Eaton myasthenic syndrome, also referred to as the myasthenic syndrome, affects primarily adults older than 40 years of age, with a slight predilection to men. Patients present with proximal muscle weakness (especially of the lower extremities) and minimal ocular and bulbar weakness, and are susceptible to fatigue. Deep tendon reflexes are characteristically absent or reduced. Autonomic complaints (especially dry mouth) and transient paresthesias may also occur. A helpful and distinctive clinical finding is muscle facilitation: after a brief period (∼10 seconds) of intensive exercise of a muscle, muscle power is much transiently stronger and the deep tendon reflex to that muscle is enhanced. Unfortunately, this sign cannot always be confirmed during bedside evaluation. Figures C21-3 and C21-4 show the common signs and symptoms of LEMS patients, based on series of 50 patients.
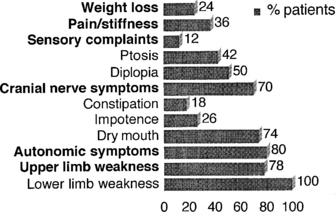
Figure C21-3 Lambert-Eaton myasthenic syndrome. Symptoms during the course of illness in 50 cases.
(Adapted from O’Neil JH, Murray NMF, Newsom-Davis J. The Lambert-Eaton myasthenic syndrome: a review of 50 cases. Brain 1988:11:577–596.)
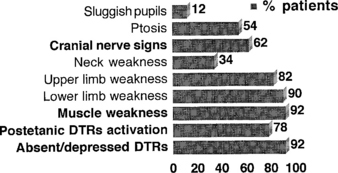
Figure C21-4 Lambert-Eaton myasthenic syndrome. Signs during the course of illness in 50 cases.
(Adapted from O’Neil JH, Murray NMF, Newsom-Davis J. The Lambert-Eaton myasthenic syndrome: a review of 50 cases. Brain 1988;11:577–596.)
Treatment of LEMS is difficult. Treatment of the primary cancer is essential but seldom results in improvement of the neurologic symptoms. Figure C21-5 outlines a practical algorithmic treatment plan of weakness for patients with LEMS. Measures to combat weakness include:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree