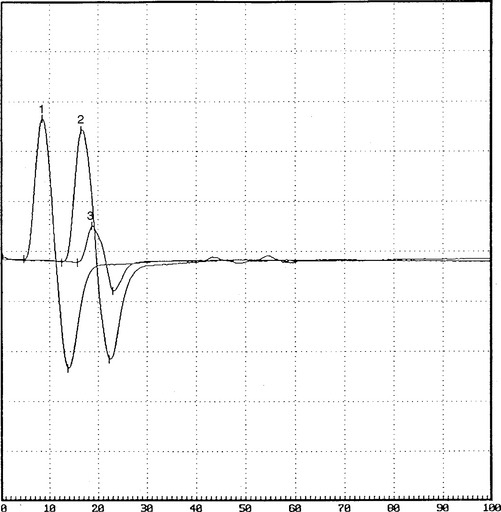
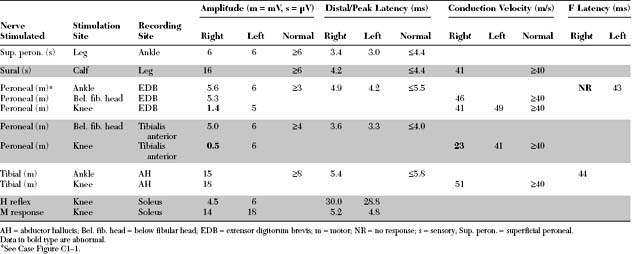
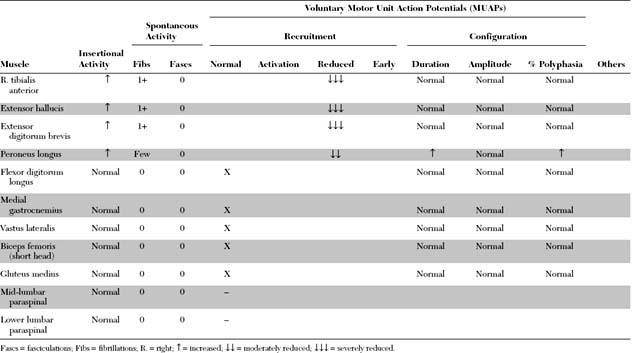
Case 1
HISTORY AND PHYSICAL EXAMINATION
EMG examination was performed 4 weeks after the onset of acute footdrop.
Please now review the Nerve Conduction Studies and Needle EMG tables.
QUESTIONS
EDX FINDINGS AND INTERPRETATION OF DATA
The pertinent electrodiagnostic EDX features in this case include the following:
DISCUSSION
Structure of Peripheral Nerve
The peripheral nerve consists of both unmyelinated and myelinated fibers and their supporting elements. The unmyelinated axons are surrounded only by the plasma membrane of a Schwann cell. The myelinated axons are engulfed by a Schwann cell that wraps around the axons multiple times, thereby insulating the axon with multiple layers of cell membrane, which is rich in lipid sphingomyelin. The myelinated axon is surrounded completely by myelin and Schwann cells, except at certain gaps. In adults, the gaps between myelin segments, called the nodes of Ranvier, measure approximately 1 μm, while myelinated segments between nodes, called the internodal segments, measure approximately 1 mm each.
Pathology of Peripheral Nerve Injury
Nerve injuries that are associated with focal interruption of the continuity of the axons cause significant changes in the structure of the peripheral nerve distal to the lesion (Table C1-1). The distal axons undergo a degenerative process, known as wallerian degeneration. This occurs since all the necessary building blocks needed for maintaining the axon are made in the cell body (peikaryon) and cannot reach the distal stump. The rate at which wallerian degeneration proceeds varies depending on the nerve injured, axon diameter, and the length of distal stump (the larger and the longer the distal stump the more time is needed for wallerian degeneration to be completed). Within hours of most nerve injuries, myelin begins to retract from the axons at the nodes of Ranvier. This is followed by swelling of the distal nerve segment, leakage of axoplasm, and subsequently the disappearance of neurofibrils. Within days, the axon and myelin fragment, and digestion of nerve components starts. By the end of the first week, the axon and myelin become fully digested and Schwann cells start to bridge the gap between the two nerve segments. In chronic nerve lesions, the endoneurial tubes in the distal stump shrink, the nerve fascicles atrophy distal to the lesion, and, in complete nerve transection, the severed ends retract away from each other.
Table C1-1 Consequences of Focal Axonal Injury Distal to the Lesion
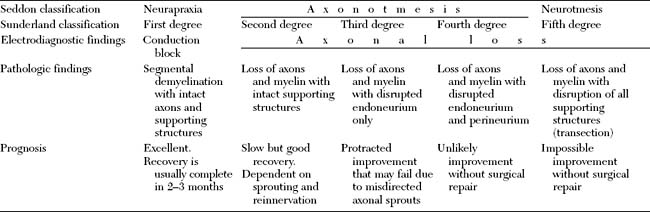
Classification of Peripheral Nerve Injury
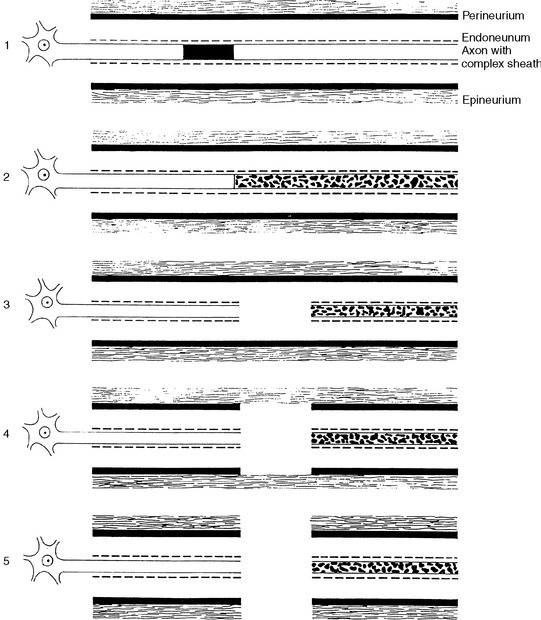
Many classifications of peripheral nerve injury have been suggested, but Seddon’s and Sunderland’s classifications are the most widely used in clinical practice. These are based on the functional status of the nerve and on histologic findings. They are shown in Table C1-2 and in Figure C1-2, with their corresponding electrophysiologic findings.
Electrodiagnosis of Peripheral Nerve Injury
The EDX studies are the cornerstone in the diagnosis and management of nerve injuries by providing valuable information as to the location of the lesion, and its severity, pathophysiology, and prognosis (Table C1-3). Intraoperatively, the EDX studies guide the surgeon during the procedure and help assess the status of the regenerating axons within the injured nerve segment. During the recovery stage of peripheral nerve injury that may occur spontaneously or after surgical repair, the EDX studies are also essential in the evaluation of remyelination, regeneration, and reinnervation.
Table C1-3 Role of Electrodiagnostic Studies in Peripheral Nerve Injury
Localization of Nerve Lesions Using Nerve Conduction Studies
Focal Slowing
Focal slowing of conduction usually is caused by widening of the nodes of Ranvier (paranodal demyelination) and, sometimes, focal axonal narrowing. It is evident on NCSs by slowing of conduction of a specific nerve segment, while other segments of the same nerve as well as neighboring nerves remain normal. When the large myelinated fibers are slowed to essentially the same extent, focal slowing across the involved nerve segment is synchronized. This is manifested by either a prolongation of distal latencies (in distal lesions) or slowing in conduction velocities (in proximal lesions), while the CMAP amplitude, duration, and area are not affected and do not change when the nerve is stimulated proximal to the lesion. When variable number of the medium or small nerve fibers (average or slower conducting axons) are affected only, desynchronized (differential) slowing of conduction across the nerve segment is evident. In this situation, the CMAP is dispersed on stimulation proximal to the lesion and has prolonged duration, with normal (nondispersed) response on distal stimulation. If this finding is isolated, the distal latency or conduction velocity, which represent the speed of the largest (fastest) axons, are normal. However, in most clinical situations, the large fibers are often involved also, desynchronized slowing is usually accompanied by slowing at the involved segment, resulting in concomitant slowing of distal latency or conduction velocity.
Conduction Block
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree