■ Arterial blood pressure monitoring is necessary for optimal anesthetic management. Bladder catheterization is performed if the procedure is expected to extend beyond 2 hours. If endarterectomy is performed with regional anesthesia, an audible squeeze device is placed in the patient’s contralateral hand for indirect neurologic monitoring. Preoperative antibiotics are administered routinely.
■ Aspirin therapy is initiated well in advance of surgery and continued throughout the perioperative period. Evidence suggests that statin therapy, initiated preoperatively, reduces postoperative neurologic events and mortality.7
TECHNIQUES
CAROTID ENDARTERECTOMY—PATCH ANGIOPLASTY
Incision
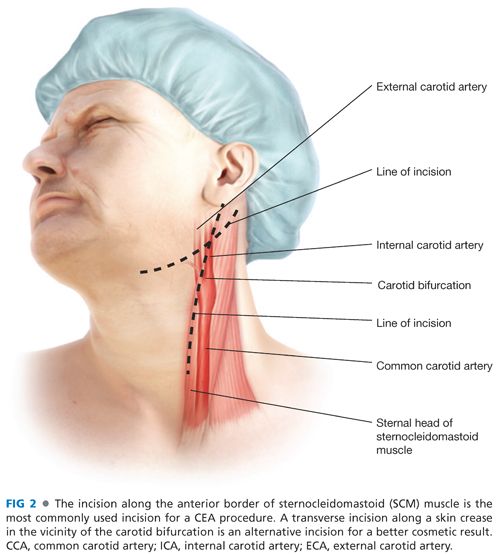
■ The skin incision is optimally placed along the anterior border of the sternocleidomastoid muscle. This should be curved posterolaterally near the angle of the mandible to avoid dissection into the parotid gland.
■ Alternatively, a more transverse incision can be made at the level of the carotid bifurcation. Although providing an improved cosmetic result, exposure of the distal ICA may be compromised with this approach (FIG 2).
Carotid Exposure and Control
■ As the incision is extended through the platysma muscle, the anterior border of the sternocleidomastoid muscle is visualized and retracted posterolaterally. The greater auricular nerve should be identified and protected at the superior extent of the incision.
■ Following fascial incision, the facial vein is identified and securely ligated. This vein usually transverses the CCA near the bifurcation. Failure to adequately secure this vein may lead to bleeding and airway compromise during postoperative cough spells or Valsalva maneuvers.
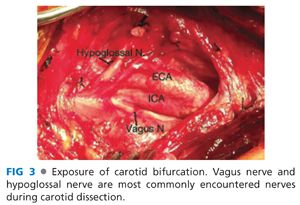
■ Within the carotid sheath, the vagus nerve usually extends posterior to, and parallel with, the artery and vein. However, this position relative to the other contents of the carotid sheath may vary, and the vagus should always be identified and protected in the course of the dissection. The ansa cervicalis nerve is commonly much smaller than the vagus and runs anterior to the carotid bifurcation. When completely isolated, the proximal ansa arises from the ipsilateral hypoglossal (XII) cranial nerve. The ansa cervicalis can be divided to improve exposure if necessary or mobilized sufficiently to be gently retracted out of the operative field.
■ The CCA is circumferentially dissected from surrounding structures in sufficient length to provide adequate exposure for proximal clamping and control. The CCA is optimally controlled by placement of an appropriately sized, atraumatic vascular clamp such as a Gregory profunda clamp. The ratchet should be engaged only to the minimal amount necessary to control bleeding to prevent intimal injury and dissection at the site of clamp placement.
■ Following common carotid control, the dissection is extended cranially and posteriorly along the posterolateral border of the ICA. Development of the dissection plane posterolaterally along the proximal ICA minimizes risk of hypoglossal nerve injury. This dissection is also performed with minimal displacement and instrumentation of the ICA to reduce intraoperative embolization risk (FIG 3).
■ To complete the necessary exposure, the external carotid artery (ECA) is dissected and mobilized to at least the level of the superior thyroidal artery. The superior laryngeal nerve may also be encountered posterior to the carotid bifurcation in this area.
■ Following dissection, and prior to clamp placement, sufficient unfractionated heparin is administered intravenously to obtain an activated clotting time (ACT) of more than 200 seconds. With normal circulation times, this is usually accomplished within 2 or 3 minutes of injection.
■ Clamping of ICA is performed first, followed by control of the external and common carotid arteries. This sequence is followed to minimize embolization risk associated with clamping. When necessary, measurement of ICA stump pressure is obtained at this juncture by cannulation of the carotid bifurcation and selective removal of the internal carotid clamp.
Conventional Endarterectomy
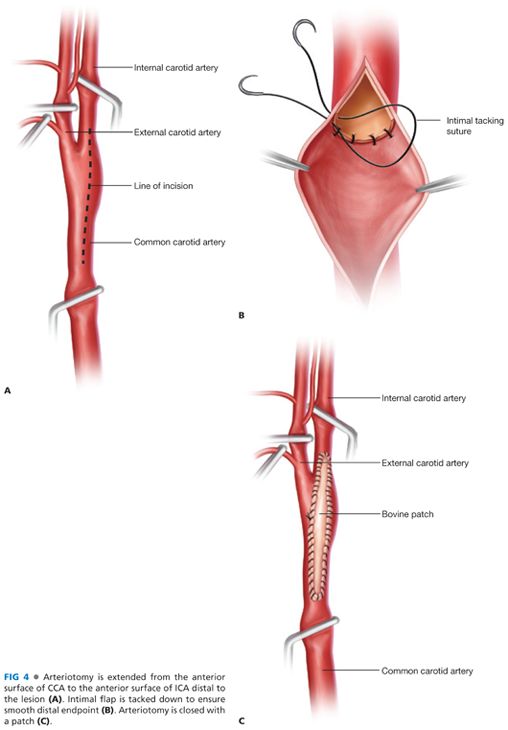
■ The arteriotomy is initiated in a soft, uninvolved proximal segment of the CCA and extended cephalad with Potts scissors. It should be positioned on the anterior-lateral surface of the ICA to avoid the flow divider. (FIG 4A).
■ When an indwelling shunt is indicated or required, the distal tapered end is carefully inserted into the ICA under direct vision. We prefer the Pruitt-Inahara shunt, which has pilot balloons at both ends to maintain shunt position and hemostasis. Once the distal end is inserted, the distal balloon is inflated with less than 1 mL of air until the “pop-off” balloon inflates on the pilot tube. Familiarity with this shunt prior to insertion is essential; if the inflation override cuff covers the “pop-off” balloon on the pilot tube, overinflation may injure or rupture the distal ICA. Following distal ICA cannulation and balloon inflation, the shunt is back-bled to confirm luminal placement and decant air. With the shunt actively back-bleeding, the proximal end is inserted into the CCA followed by proximal clamp removal into the unobstructed lumen. The proximal pilot tube is inflated with the provided syringe until the cuff is palpable in the CCA, after which a prepositioned Rumel tourniquet is gently cinched around the artery. When performed quickly, with concurrent digital control of the CCA following clamp removal and prior to shunt insertion, minimal bleeding ensues. When saline is applied to the shunt tubing, pulsatile flow is appreciable with handheld Doppler insonation.
■ At the site of maximal atherosclerotic disease in the CCA, the Penfield knife is employed to identify and develop the appropriate endarterectomy plane within the medial layer. When the correct plane is identified, the plaque is easily and rapidly elevated from the underlying adventitia. In areas containing intraplaque hemorrhage, inflammation may increase adherence of the plaque to the adventitia, and care should be taken not to extend the dissection plane into the adventitia itself.
■ At the distal extent plaque, sufficient exposure should be present to create a defined endpoint, allowing placement of tacking sutures if necessary, ensuring that no further potentially mobile plaque remains. It is essential to “feather” the plaque at the distal endpoint to minimize risk for distal dissection or thrombus accumulation. If the plaque extends past the point where feathering is feasible, a distal endpoint should be determined and created sharply with scissors or a no. 15 blade (FIG 4B). Tacking sutures, placed circumferentially, can control distal plaque at the transection site. Care should be taken, however, to place the minimal number of sutures necessary to prevent dissection, or consider extending the arteriotomy and endarterectomy to identify a more suitable termination site. Successful suture placement requires circumferential dissection and optimal visualization.
■ Once the distal endpoint is determined, residual plaque is removed from the ECA by eversion into the CCA and circumferential dissection and traction. Sufficient back-bleeding is performed to remove any luminal debris within the ECA.
■ Direct visualization of the endarterectomy bed following plaque removal commonly identifies loosely attached residual medial elements. These are best removed with fine forceps under magnification. Complete removal is facilitated by continuous irrigation to identify mobile medial elements. Integrity of the distal and proximal endpoints is also verified using this technique.
Patch Placement
■ An appropriately sized bovine pericardial or Finesse Dacron knitted polyester patch is selected and trimmed as necessary for closure-assisted angioplasty. Both bovine pericardial and polyester patches have chirality considerations; one surface is preferred for luminal apposition. Please consult the accompanying instructions for use prior to implantation. Closure is secured with running 6-0 polypropylene suture initiated at the cephalad extent of the arteriotomy and continued proximally along the long axis of the patch.
■ After 90% or more of the circumference of the patch is secured, flushing is accomplished by sequential clamp removal and luminal irrigation with heparinized saline. Closure is then completed prior to restoration of flow.
■ The declamping sequence is of critical importance. The CCA is released first, followed by the ECA clamp. After several cardiac cycles have ensued, the distal ICA is released (FIG 4C).
■ We perform intraoperative completion duplex imaging of the endarterectomy site as well as the proximal and distal carotid arteries, with purpose-designed, miniaturized 7 MHz probes. Completion duplex scanning is quick, efficient, highly reproducible, and effective at identifying significant residual luminal defects. Detailed description of the characteristics of significant luminal defects identified by completion ultrasonography are beyond the scope of this chapter. Intraoperative insonation is not possible through extruded polytetrafluoroethylene (ePTFE) patches and should not be attempted.
Closure
■ Following adequate duplex imaging and endpoint determination, anticoagulation is reversed with protamine sulfate. Some practitioners are reluctant to reverse anticoagulation due to uncertainty regarding thrombogenicity at the endarterectomy site. In our experience, technical issues at the endarterectomy site are most predictive of postoperative neurologic events, and these are efficiently identified and corrected, when present, with completion ultrasonography. Following reversal, the entire wound is inspected for venous or arterial bleeding. The entirety of the patch angioplasty suture line is reinspected for periodicity of suture placement and potential leaks. Reinforcing sutures are applied liberally as needed to ensure hemostasis, but with experience and even suture spacing, the need for additional sutures should be rare. Bleeding lymph nodes should be sutured and removed from the operative field. Confirmation of hemostasis, the platysma is reapproximated with running absorbable suture followed by skin closure. We usually also perform a Valsalva maneuver to identify occult venous injuries that may not be apparent with positive pressure ventilation prior to closure.
CAROTID ENDARTERECTOMY—EVERSION
Incision
■ See section under Carotid Endarterectomy—Patch Angioplasty.
Dissection and Control of the Carotid Artery
■ See section under Carotid Endarterectomy—Patch Angioplasty.
Eversion endarterectomy
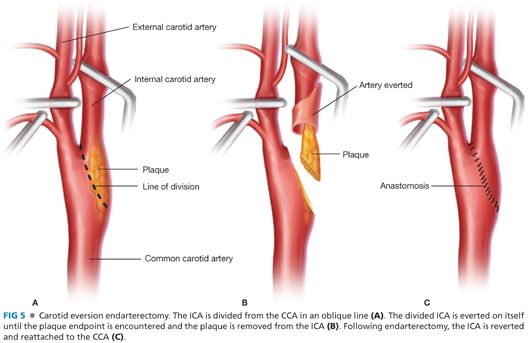
■ An oblique or circumferential incision is made at the junction of the bulbous portion of the ICA and CCA (FIG 5A).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


