FIGURE 22-1 From the transverse aorta, the Simmons 2 catheter is prolapsed into the ascending aorta with counterclockwise rotation creating a loop on itself that will select the left common carotid artery. A 0.025-inch straight glidewire assists with selection.
Alternative catheters for left carotid angiography may also be used. A Simmons 1 diagnostic catheter works well when the distance between the innominate and the left common carotid is narrow, while a Tiger or Kimny catheter is better for wider distances. A 0.025-inch glidewire will not deform the primary curve of the diagnostic catheter and is useful in selecting the common carotid.
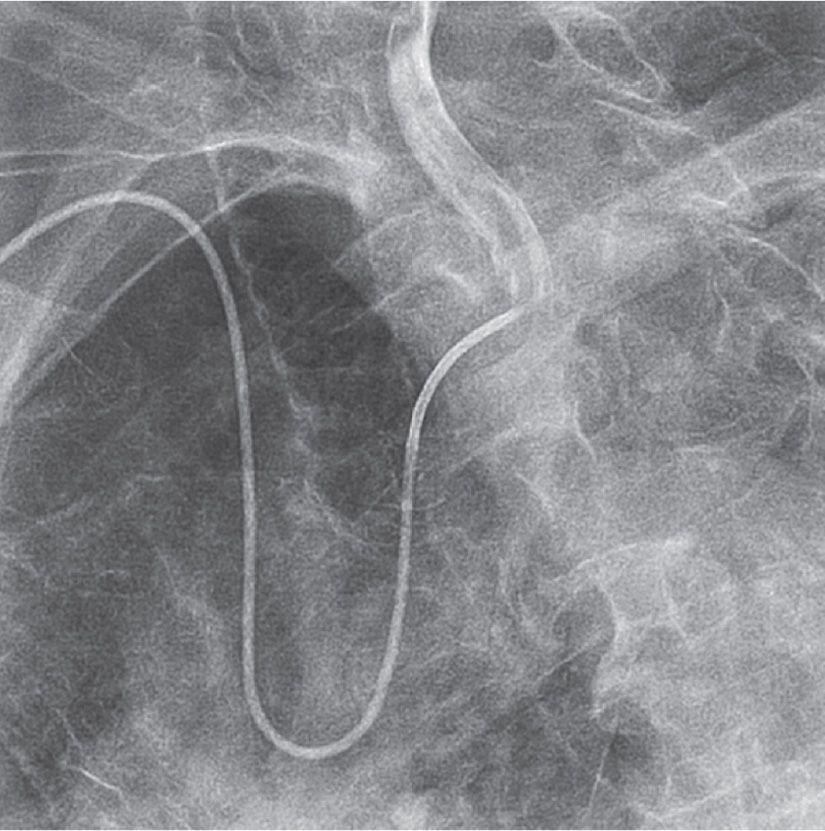
FIGURE 22-2 A Simmons 2 catheter positioned in the left common carotid artery.
In the presence of bovine left carotid anatomy, the right radial approach is the preferred strategy for angiography and stenting. An Amplatz AR2 will easily select the origin of the bovine common carotid (Fig. 22-3).
For TR right carotid angiography, catheter selection is based on an initial RAO subclavian arteriogram (Fig. 22-4). The latter defines subclavian tortuosity as well as the angle of takeoff of the right common carotid artery. A Simmons 1 catheter is used in most cases (Fig. 22-5). A Tiger catheter is an alternative choice, especially when the angle of takeoff of the right common carotid is less acute.
Right Radial Access for Carotid Stenting
It is apparent from initial feasibility studies that the right TR technique for carotid stenting involves three different techniques for the three basic anatomical types of carotid disease: right internal carotid, bovine left internal carotid, and nonbovine left internal carotid. Thus, this section is subdivided to review the three basic techniques.
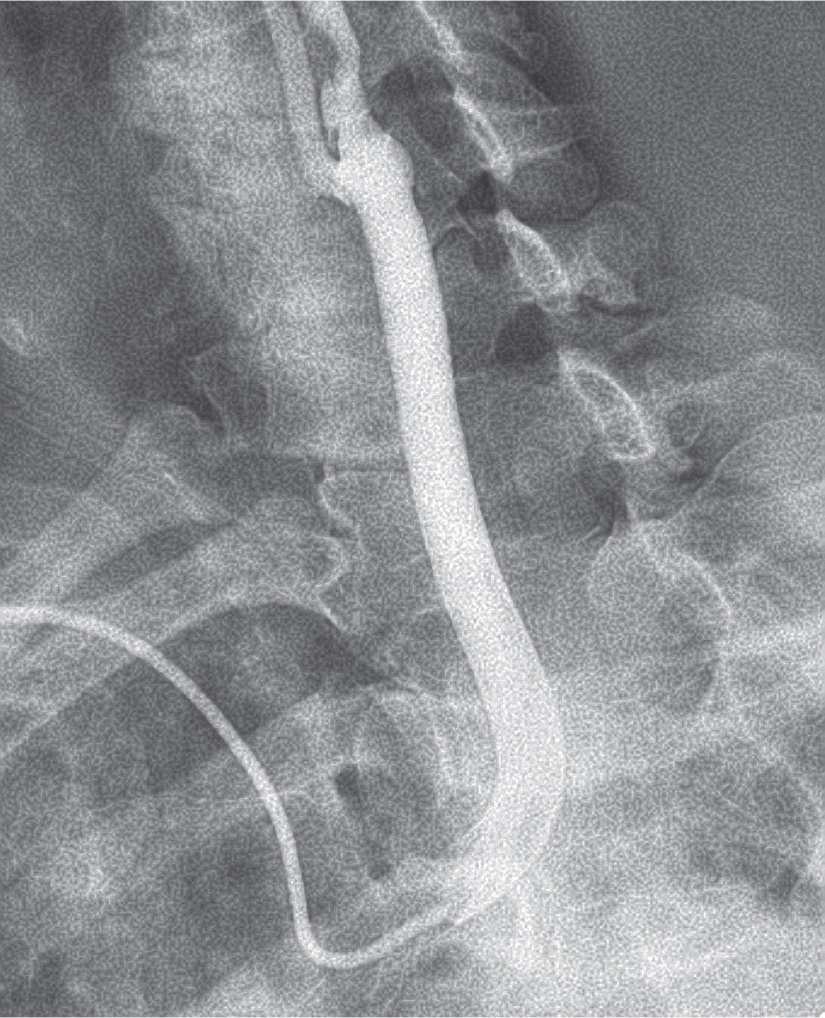
FIGURE 22-3 The bovine left carotid is selected with an Amplatz AR2 catheter.
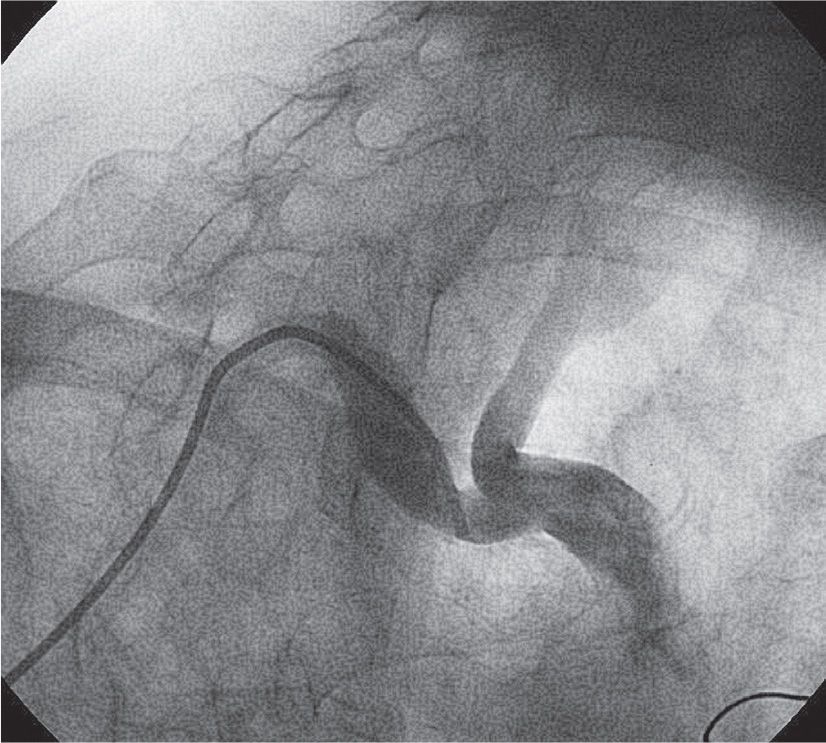
FIGURE 22-4 An RAO arteriogram of the innominate artery bifurcation.
The technique of TR insertion of a sheath for carotid stent deployment is a variation of the technique used for femoral access. Thus, a diagnostic catheter positioned in the external carotid artery is used for the insertion of an exchange-length, supportive guidewire over which the sheath is deployed. Once the sheath is in place, the technique of embolic protection device deployment and carotid stenting is precisely the same as from the femoral artery. However, deployment of the sheath from the right arm necessarily involves acute angles that must be negotiated, and different catheters are required than the ones used with the femoral approach.
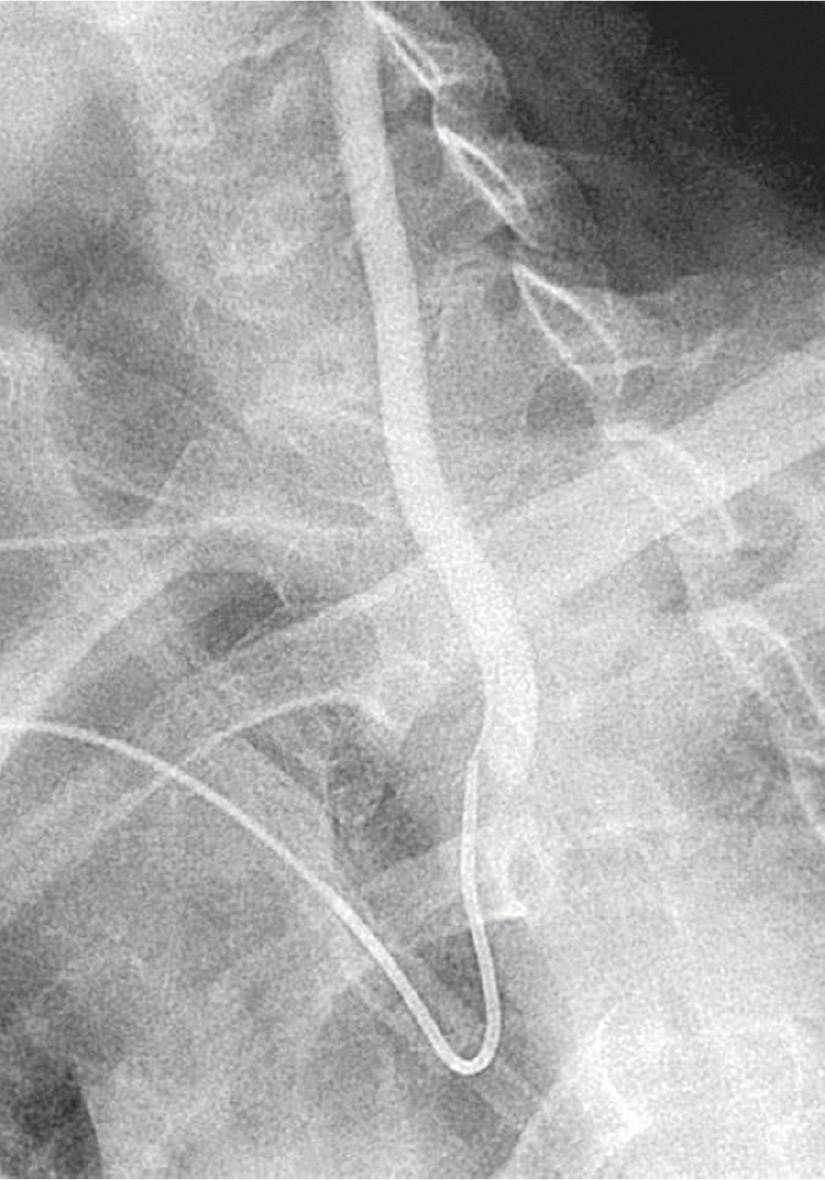
FIGURE 22-5 Simmons 1 catheter positioned in the right common carotid artery.
Right Internal Carotid
The right carotid is selected with a 5F Simmons 1 diagnostic catheter. The original technique of right internal carotid artery stenting involves passing the Simmons 1 catheter into the external carotid artery over a glidewire or extra-support coronary guidewire (Fig. 22-6).10 An exchange-length 0.035-inch J wire was then inserted, which serves as the platform for sheath deployment. Although an Amplatz superstiff wire is used for this purpose from the femoral approach, excessive tension is created by the acute angles from the arm and its use should generally be avoided.
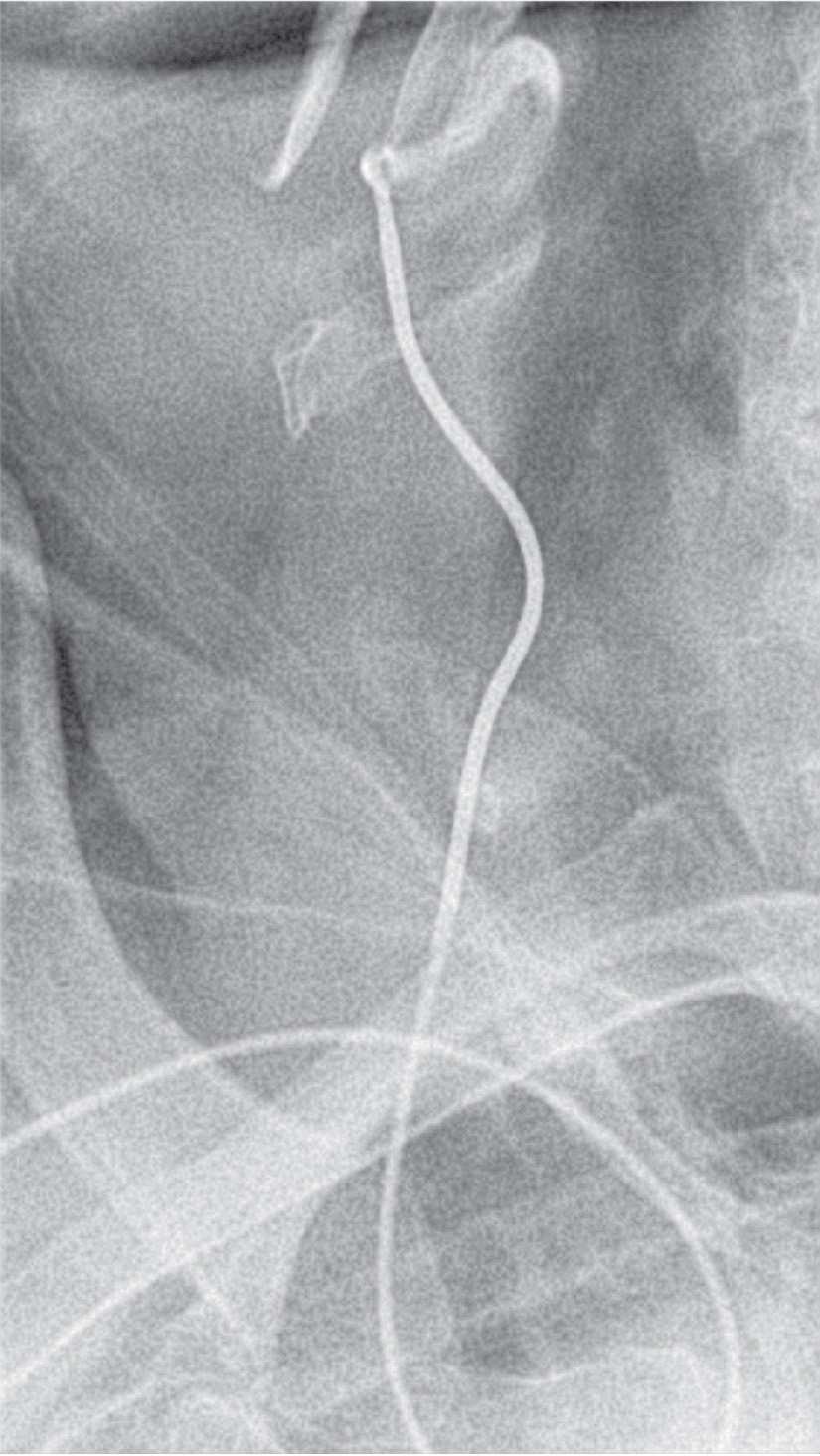
FIGURE 22-6 French Simmons 1 catheter in right external carotid artery.
The TAD II (Covidien, Mansfield, MA) is a 0.035-inch exchange-length guidewire that tapers to a soft 0.018-inch floppy tip. The external carotid can often be directly cannulated with this guidewire, which then serves as a platform for the deployment of the shuttle sheath. Thus, the step of passing the diagnostic catheter into the external carotid artery is omitted, expediting the procedure. The Advantage (Terumo Corporation, Somerset, NJ) hybrid wire may also be used for this purpose, and the selection of the external carotid with its hydrophilic tip may be easier.
When the angle of takeoff of the right common carotid is vertical or acute (Fig. 22-7), a telescopic technique with the sheath (90 cm) over the Simmons 1 catheter (100 cm) is useful to traverse the acute angle. A 5F shuttle sheath is best used in this situation in view of its flexibility and smooth transition with no lip at the sheath/catheter junction.
An alternative strategy for right carotid acute angles involves the use of a wire-braided Simmons 2 catheter (see the previous paragraph for reforming technique). This catheter is passed into the external over a glidewire or extra-support coronary wire and then exchanged for the shuttle sheath. This catheter should not be used in women or short patients as the long distal limb may actually reach the carotid bifurcation.
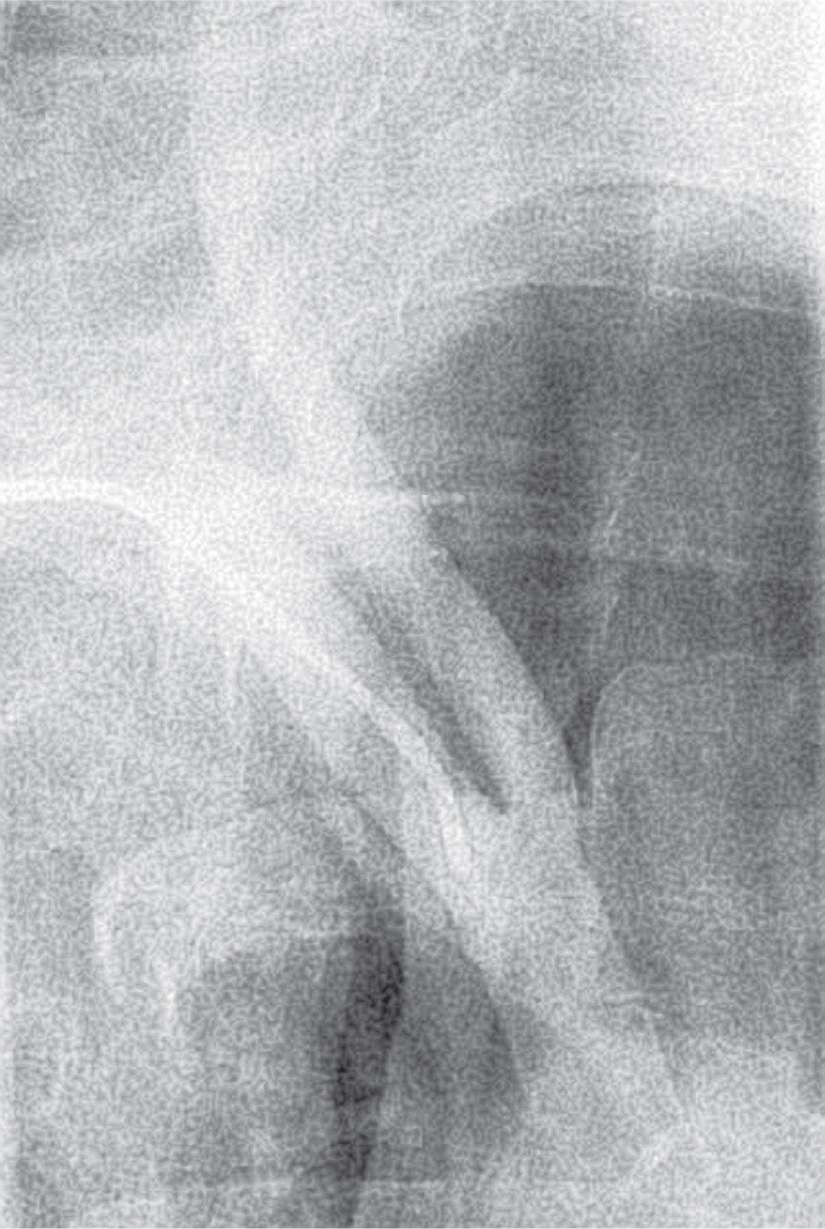
FIGURE 22-7 An RAO innominate arteriogram demonstrating a vertical takeoff of the right common carotid with no horizontal segment for support.
When a small radial artery or acute angle necessitates the use of a 5F shuttle sheath, only lower-profile Precise stents and Wallstents are compatible. An important caveat is that suction produced by the introduction of stent can introduce air into the system. Thus, stents are positioned with roadmap fluoroscopy and/or bony landmarks. Alternatively, one can patiently wait for complete bleed-back before injecting the contrast material.
Nonbovine Left Internal Carotid
The nemesis for the development of a consistent technique for nonbovine left internal carotid stenting from the right radial artery has been the acute angle that must be traversed for the deployment of a shuttle sheath in the left common carotid. The angle results in poor inferior support for the catheter system, resulting in a tendency for catheters to prolapse into the ascending aorta. This problem is particularly ominous should prolapse occur during stent delivery.
The use of a wire-braided Simmons 2 catheter (Cook Medical, Bloomington, IN) with its relatively long distal end has been found to provide adequate support for passing a guidewire into the external carotid in the majority of cases. A Simmons 3 catheter provides even more support and is utilized in cases of type II and type III arches with particularly acute angles of takeoff of the common carotid.
The anchoring technique with a stepwise exchange of glidewire or TAD II wire and 0.035-inch J wire through the diagnostic catheter in the external is most commonly utilized. With extreme angles, a 5F or 6F multipurpose or Amplatz R2 guide catheter can be used as an intermediate transfer catheter to insert an Amplatz superstiff guidewire. In these extreme situations, kinking of the shuttle sheath may occur at the acute angle, and a heavily braided, semihydrophilic Destination sheath (Terumo Corporation, Somerset, NJ) should be substituted.
A telescopic approach with a Simmons 2 diagnostic within the sheath is preferred in most cases of nonbovine left internal carotid artery (LICA) lesions. As previously noted, a 5F shuttle sheath is more flexible without a lip at the sheath/catheter transition. If a 6F sheath is required, it should be telescoped over a 6F diagnostic Simmons 2/3 to prevent this transition lip from catching at the origin of the common carotid.
In patients with a type I arch and left internal carotid lesions, the TAD II guidewire can be passed directly into the external and usually provides sufficient support for the deployment of the shuttle sheath (Fig. 22-8). Again, a telescoped 5F shuttle sheath/Simmons 2 is better. This simple technique is successful only with less severe angles of takeoff of the left common carotid.
The catheter looping and retrograde engagement technique (CLARET) is an alternative strategy that has been described by Fang et al.17 in a large series of patients. The unique feature of this technique is the use of the right coronary cusp to provide inferior support for the system (Fig. 22-6). The technique uses a 7F Kimny or multipurpose guide catheter placed in the carotid for the stent procedure. Because of the large external diameter of this catheter, it can be used only in a minority of patients transradially unless inserted sheathlessly. Catheter instability also may be problematic.
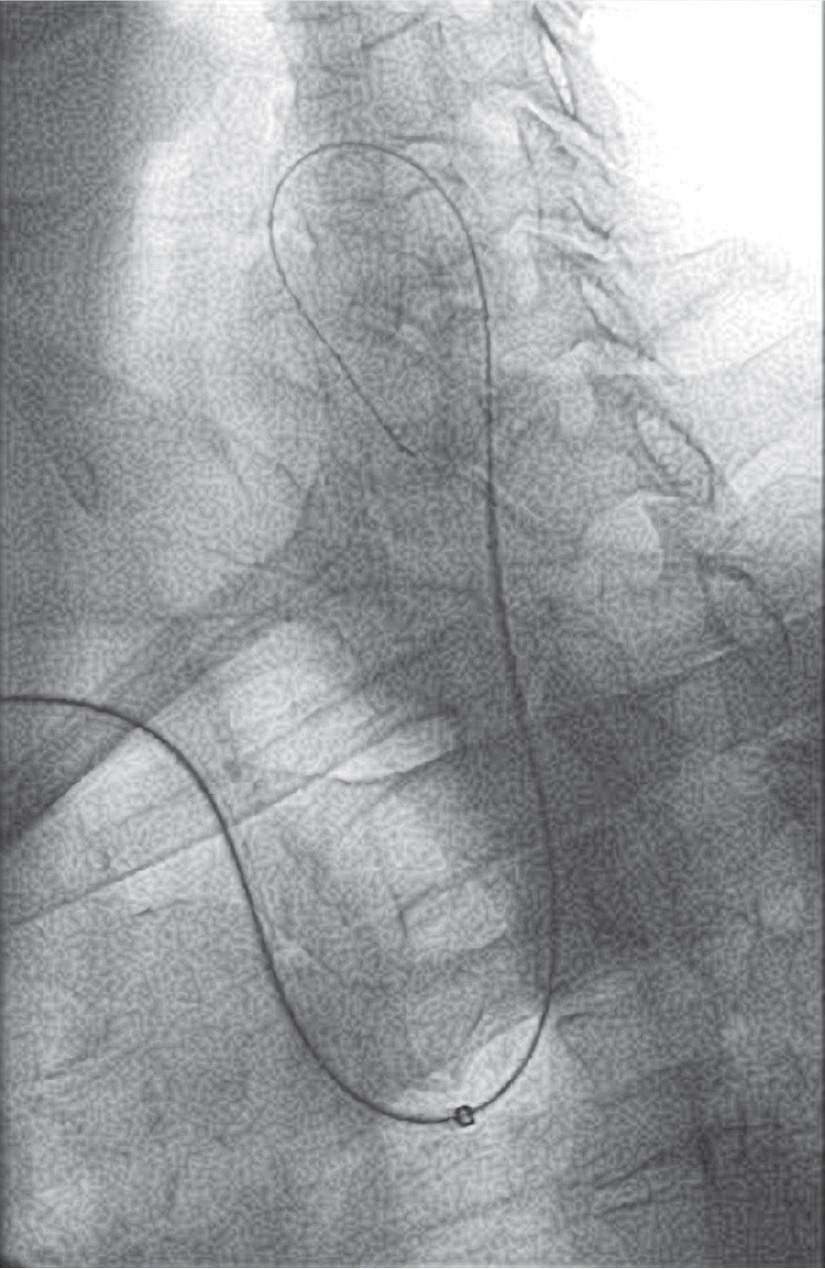
FIGURE 22-8 A shuttle sheath telescoped over a Simmons 2 diagnostic catheter introduced over a TA D II guidewire into the left internal carotid artery in a patient with a type I arch.
Bovine Left Internal Carotid
The right TR approach may be the preferred strategy for carotid stenting of bovine left internal carotid lesions. The initial takeoff of the bovine carotid is evaluated using an LAO aortic arch arteriogram. When the initial segment of the common carotid is horizontal (Fig. 22-3), an Amplatz R2 diagnostic catheter is suitable. When the initial segment is more vertical (Fig. 22-4), a Simmons 1 or 2 is necessary to implement the exchange for the shuttle sheath. Techniques similar to the right or nonbovine left internal carotid cases for the insertion of the shuttle sheath are utilized.
Caveats
It should be emphasized that experience in the TR approach is important as these are advanced techniques. New operators are encouraged to be comfortable both with TR cerebrovascular angiography and with TF carotid stenting before undertaking these procedures.
The most important limitation of this technique is the lack of inferior support at the origin of the common carotid where sheath angulation is most extreme. This is particularly an issue with nonbovine left carotids and is the cause of most TR carotid failures. Prolapse of the shuttle sheath into the ascending aorta is most likely to occur during the initial delivery attempt, but may also occur during delivery of rigid, bulky stents. The latter situation is ominous since the embolic protection device may be pulled back across the carotid lesion while still fully deployed, increasing the risk of embolic stroke.
Finally, air introduction may occur when bulky stents are advanced through small-diameter sheaths, and stents should be positioned using roadmap fluoroscopy or bony landmarks. Contrast injections are made only after careful bleed-back.
Conclusion
The TR approach for carotid stenting is feasible and holds promise as an alternative technique to conventional femoral access. High success rates with low complications have been demonstrated with right and left carotid stenting. It is the preferred access site for bovine left carotid stenting and right carotid stenting in the presence of a “hostile” arch. However, further refinement of the technique as well as future technological improvements are necessary to simplify the procedure and optimize results.
Aorto-Iliac
John T. Coppola and Ravikiran Korabathina
Natural History
The prevalence of lower extremity peripheral artery disease (PAD) varies depending on the population studied and the criteria used to define disease. When an ankle-brachial index (ABI) measurement of <0.9 is the diagnostic standard, the overall prevalence of PAD according to the Atherosclerosis Risk in Communities study cohort for individuals between 45 and 64 years of age was 2.6%.29,30 The Multi-Ethnic Study of Atherosclerosis study cohort showed a significant rise in prevalence with each subsequent age decade, rising to 6.44% in those between 65 and 74 years and 11.98% in those between 75 and 84 years.29,31 Lower extremity PAD is also highly prevalent in populations with high atherosclerotic risk factor burden, as illustrated by the PAD Awareness, Risk and Treatment: New Resources for Survival (PARTNERS) study.32 Of 6,417 patients presenting to primary care practices who were 70 years or older or aged 50 to 69 with diabetes mellitus or tobacco dependence, PAD was detected in 29%. In the population with manifest cardiovascular disease defined by a history of angina, myocardial infarction, coronary revascularization, aortic aneurysm repair, stroke, or carotid endarterectomy, the prevalence increased to over 40%. Furthermore, PAD was more common in African Americans and women without cardiovascular disease.
Aorto-iliac PAD appears to have a different epidemiology when compared to infrainguinal PAD, likely stemming from the histological differences between these arterial beds. In a retrospective analysis, atherosclerosis of the larger and more elastic aorto-iliac arteries was associated more so with smoking and to a lesser degree with diabetes.33 When looking at patients with early-onset PAD, defined as onset before the age of 50, interestingly, 65% were shown to have aorto-iliac disease in one study, and the risk factors strongly associated with this disease location were the absence of diabetes, low HDL cholesterol, and female gender.34 The relation of female gender to aorto-iliac disease is intriguing, especially given that hypoplastic aortic vessels are more common in women, and an association between smaller aortic diameters and aortic atherosclerosis progression has been demonstrated.35 Typically, PAD presents as a multilevel disease, with most surgical series reporting that up to two-thirds of individuals present with both aorto-iliac and infrainguinal disease.36 Nevertheless, the presence of large-vessel PAD has been shown to carry greater prognostic implications, with a nearly fourfold higher risk for fatal and nonfatal cardiovascular events than is seen with small-vessel PAD.37
Clinical Diagnosis and Vascular Testing
Many patients with PAD have no symptoms, and a large proportion of symptomatic individuals do not present with classic intermittent claudication.38 Typical symptoms of aorto-iliac disease include buttock, hip, and thigh pain while walking; however, patients may also present with leg fatigue, muscle weakness, erectile dysfunction, and tissue loss. As a means of uniformly describing symptoms, both the Fontaine and Rutherford classification systems have been used (Table 22-1).
Noninvasive physiologic vascular testing helps with localizing lower limb PAD. Using segmental limb pressures, a thigh-brachial index (TBI) of >1.1 is considered normal, and a value <0.9 is abnormal.39 With the placement of a high thigh cuff, an abnormal TBI in both limbs suggests aorto-iliac disease, and an abnormal TBI in one limb may allude to ipsilateral iliac disease. Plethysmography can be used to obtain pulse volume recordings (PVR). As the normal PVR is characterized by a sharp systolic upstroke and a prominent dicrotic notch during the downslope toward the baseline, when aorto-iliac disease is present, the thigh PVR will lose the dicrotic notch and become rounded and blunted (Fig. 22-9). Furthermore, all PVRs from the bilateral limb segments would be abnormal. In unilateral iliac disease, the thigh PVR in the affected limb would be abnormal with normal-appearing waveforms in the contralateral limb.
The degree of stenosis required to exert a physiologic impact large enough to produce ischemic symptoms has been defined in older angiographic surgical series. Using an experimental iliac model, one study showed that a focal 1-cm lesion of 80% would be required in order to reduce limb flow by 20%.40 Studies of symptomatic patients with claudication and intermediate lesions between 50% and 75% have shown that when an aorto-femoral peak systolic pressure gradient of 10 mmHg exists across an iliac stenosis, symptoms improve after revascularization.41 While multiple criteria for translesional gradient assessment have been described, including the use of peak systolic gradient, mean gradient, and pulse pressure differential, the peak systolic gradient has been shown to carry the highest sensitivity.42
TABLE 22-1 Classification of Peripheral Arterial Disease: Fontaine’s Stages and Rutherford’s Categories
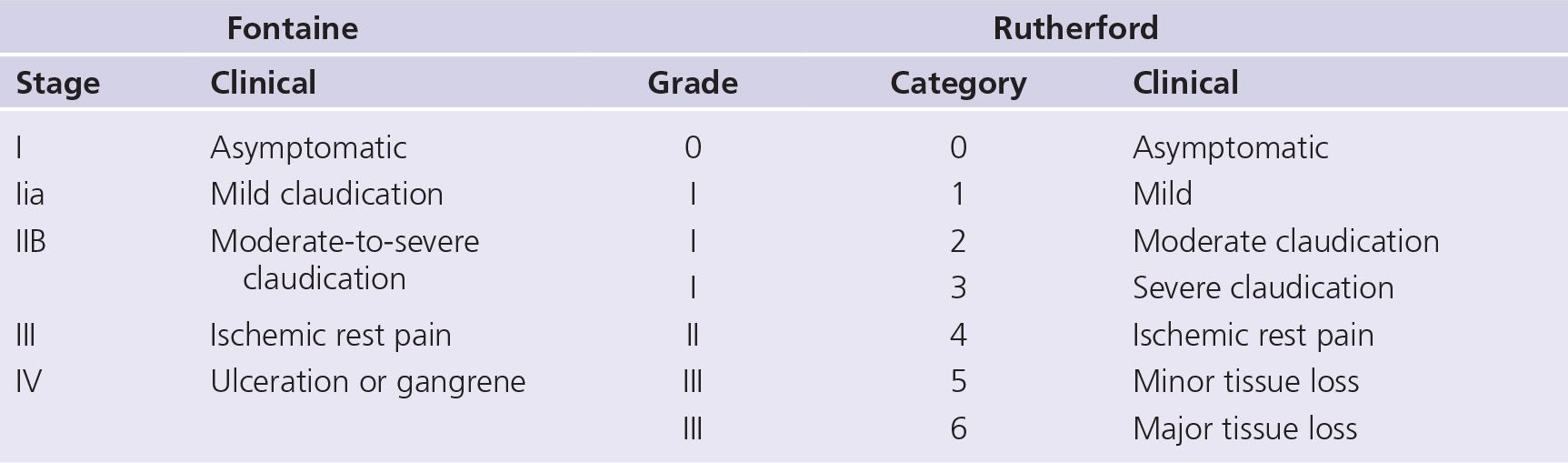
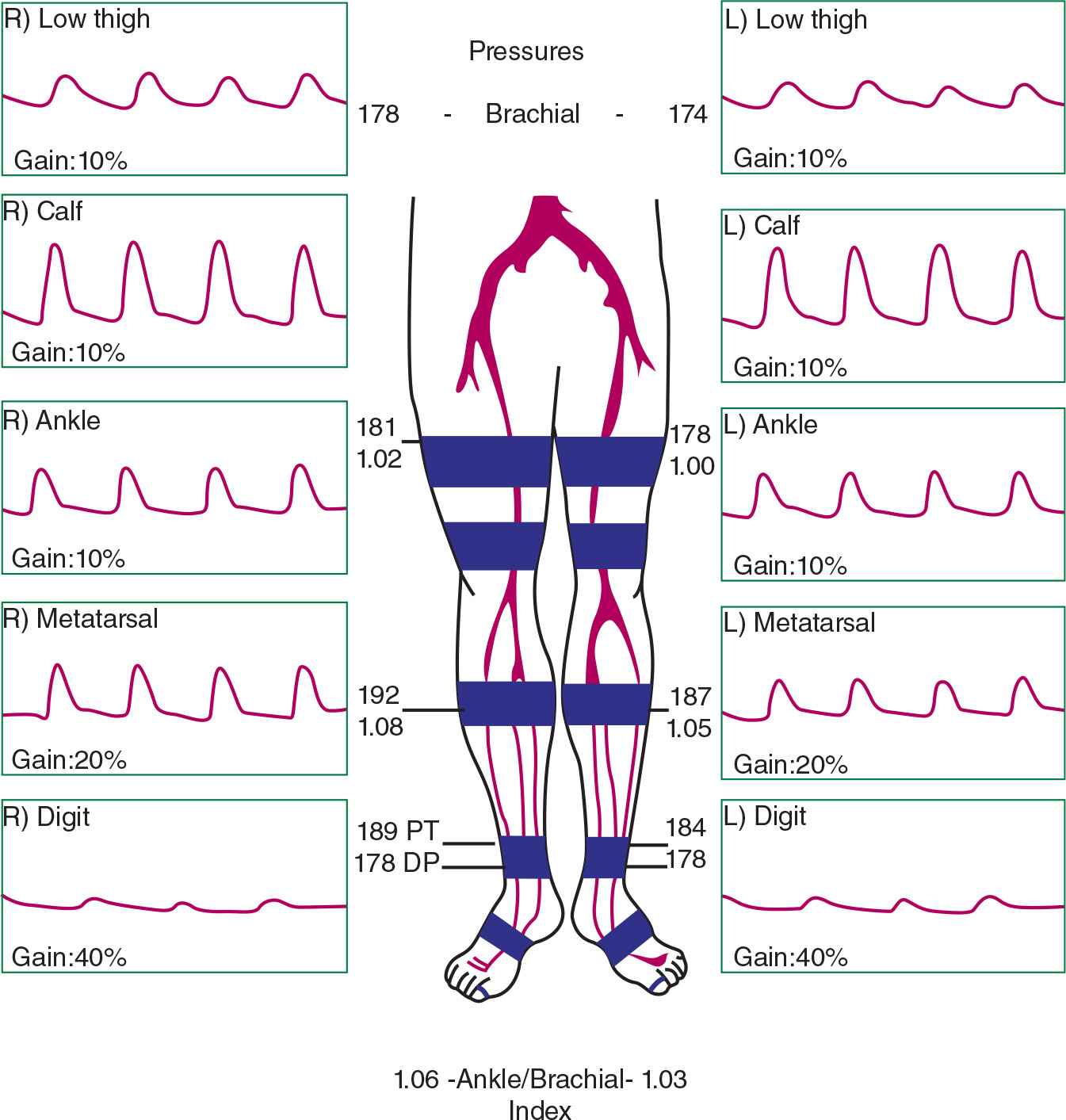
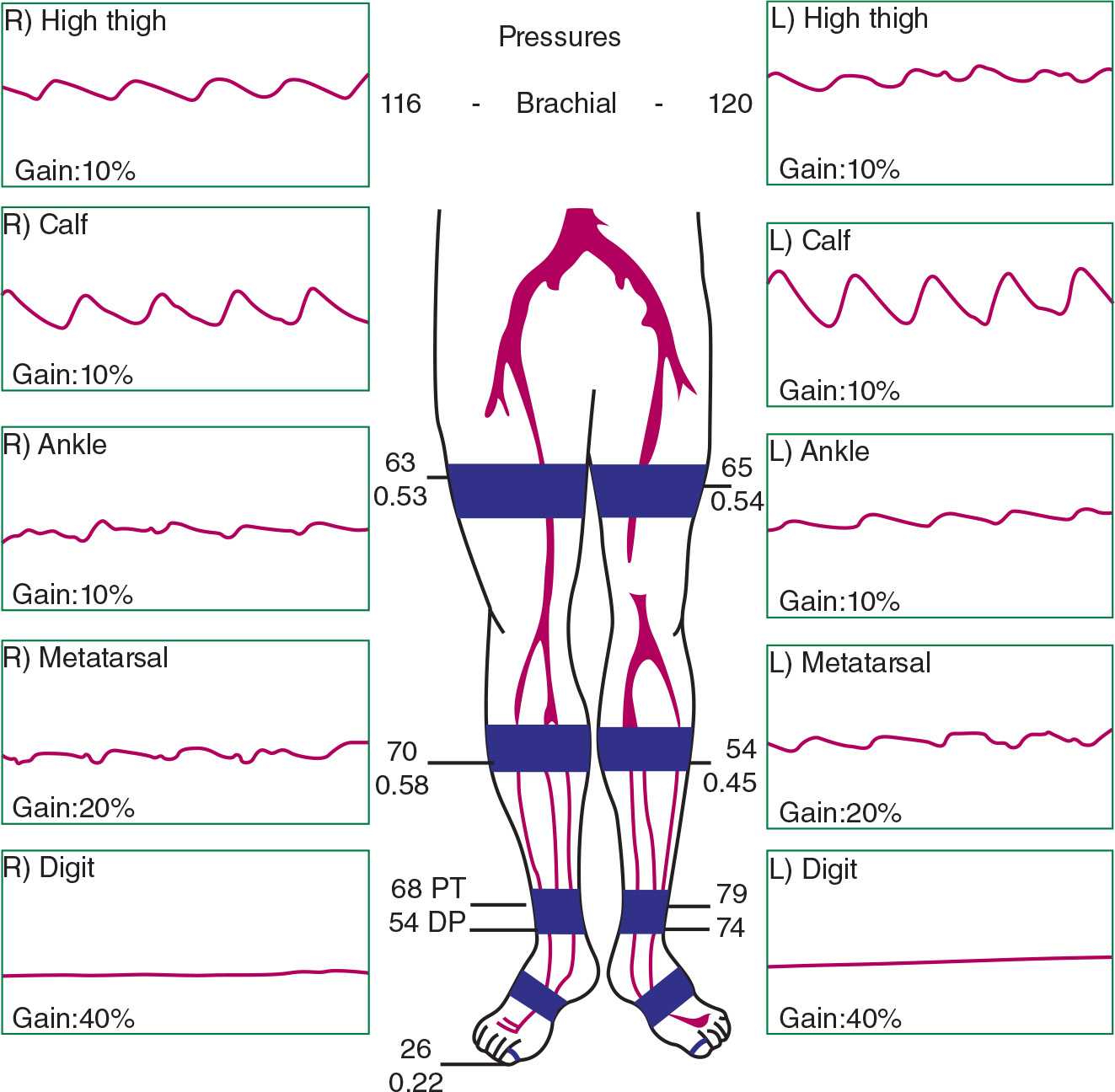
FIGURE 22-9 A: A normal PVR study. B: An abnormal PVR study in a 54-year-old female with bilateral buttock claudication with ambulation, demonstrating abnormal bilateral thigh waveforms suggesting aorto-iliac disease. (From Parks Medical Electronics, Aloha, Oregon.)
Treatment
Conservative
Medical Therapy. The treatment landscape of aorto-iliac PAD has changed markedly over the past two decades, especially since its recognition as a coronary heart disease risk equivalent. As such, aggressive atherosclerosis risk factor modification, including the appropriate introduction of lipid-lowering, antiplatelet, antihypertensive, and antihyperglycemic drugs has become the cornerstone of therapy. Smoking cessation therapies and following the dietary prescription as outlined in the National Cholesterol Education Program Adult Treatment Panel III guidelines are particularly important.43 Pharmacologic drugs directed at claudication treatment have also proven useful, and in the United States, a trial with cilostazol has been given a class I indication and pentoxifylline a class IIb indication for symptom relief and functional improvement.44
Supervised Exercise Rehabilitation. Adjunctive to medical therapy, rehabilitation therapy for intermittent claudication is fully supported by the literature. Gardner and Poehlman45 performed a meta-analysis of 21 studies, showing a marked 179% increase in distance to claudication onset and 122% increase in distance to maximal claudication pain, following a program of exercise rehabilitation. This meta-analysis also detailed the parameters that confer the most benefit being an exercise program that lasts 6 months and includes 30-minute exercise sessions occurring three times weekly. Moving a step further, Spronk et al.46 recently compared supervised exercise training to angioplasty in 151 patients with mostly aorto-iliac disease. They demonstrated equal efficacy between these two treatment strategies in terms of functional capacity and quality-of-life scores 12 months following either therapy.
The effectiveness of an initial strategy of conservative therapy for aorto-iliac PAD versus immediate revascularization is supported by randomized controlled trial data. In an older study, Whyman et al.47 compared 62 patients with ilio-femoral stenoses and claudication who were randomized to treatment by balloon angioplasty or conventional medical therapy. There was no difference in treadmill onset to claudication or maximum walking distance at 2 years. Unfortunately, the limitations of this study were the inclusion of only a small number of discrete iliac stenoses, exclusion of iliac occlusions, high screening failure rate in meeting the study criteria, and unclear but likely outdated medical therapy. Frans et al.48 conducted a meta-analysis looking only at randomized controlled trial data in the more modern era and confirmed the equivalence of supervised exercise training with percutaneous transluminal angioplasty (PTA) in terms of improved walking distances. Unfortunately, this meta-analysis also suffered limitations owing to the inclusion of both iliac and femoral lesion types and the heterogeneity of end point definitions. Most recently, short-term data from the multicenter Claudication: Exercise versus Endoluminal Revascularization (CLEVER) study have been presented.49 This trial randomized 119 patients with aorto-iliac disease, including almost 40% with iliac occlusions, to optimal medical care, supervised exercise, or stent placement. This is the only trial to date that has focused exclusively on large, proximal-vessel PAD, with three comparative arms: medical therapy alone, exercise training, and endovascular stenting. Supervised exercise therapy conferred a significantly greater change in peak walking time over 6 months when compared to either stent placement or optimal medical therapy alone.
Revascularization
When revascularization is deemed necessary, as when there is failure of conservative therapies, the patient’s comorbidity profile and lesion anatomy help in selecting the appropriate revascularization option. An anatomic classification of aorto-iliac PAD was devised by the Trans-Atlantic Inter-society Consensus Working Group (TASC II) in an attempt to balance the risk and durability of revascularization procedures and to make suggestions regarding the choice between endovascular versus surgical revascularization options (Fig. 22-10).50 Type A lesions are short stenoses that yield excellent endovascular results. Type B, C, and D lesions are either long stenoses or occlusions of increasing severity. The most challenging type D lesions are felt to give such poor endovascular results that a surgical strategy should be considered as the first choice. Data regarding treatment preference for type B and C lesions are limited, but type B lesions often give good results when approached using an endovascular strategy. Type C lesions provide good long-term results from an open surgical procedure, and this should the preferred choice unless the patient has a high operative risk.
In any regard, prior to proceeding with either surgical or endovascular treatments, imaging studies are compulsory in order to provide the blueprint for the repair strategy. Duplex ultrasonography gives both anatomic and physiologic data on stenosis severity. Magnetic resonance angiography has very high specificity and sensitivity for aorto-iliac disease and has the advantage of avoiding radiation and contrast. Computed tomographic angiography has comparatively similar sensitivity and specificity but requires the use of contrast and radiation.
Surgical Approach
Surgical bypass, mostly consisting of aorto-femoral and axillo-femoral bypasses, was formerly considered the primary revascularization route for patients with severe aorto-iliac disease, especially for TASC B–D lesions. The largest meta-analysis of surgical cases by de Vries et al.51 showed that while the 5-year long-term patency of these procedures is high at 91%, this is countered by their high operative mortality of 3.3% and aggregate morbidity of 8.3%. It is for these reasons that interest in percutaneous therapies has grown.
Endovascular Approach
Efficacy and Safety. The polyvascular nature of atherosclerosis, and its incumbent high risk for major adverse cardiac and cerebral events, that is frequently present in the individual who manifests with PAD makes surgical repair options riskier and often precludes the use of such an aggressive approach. Remarkable advancements in the endovascular armamentarium may offer the higher-risk PAD patient a better safety margin. In fact, the most recent ACC/AHA (American College of Cardiology/American Heart Association) PAD guidelines have given a class I indication for endovascular procedures if the anatomy is suitable (i.e., TASC A lesions) in a patient with lifestyle-limiting claudication that has not responded to exercise or medication.44 This recommendation is based on multiple series in the literature demonstrating the efficacy and safety of this approach. Bosch et al.,52 in a large meta-analysis of studies conducted in the 1990s, demonstrated a technical success rate for iliac stenting of 96% with a durable primary patency at 4 years of 74%. Furthermore, the 30-day mortality was low at 0.8%, with a major complication rate of 5.2%. More recently, Murphy et al.,53 in their large single-center experience of aorto-iliac stenting, showed 8-year primary and secondary patencies of 74% and 84%, respectively, with a 30-day mortality rate of 0.5%.
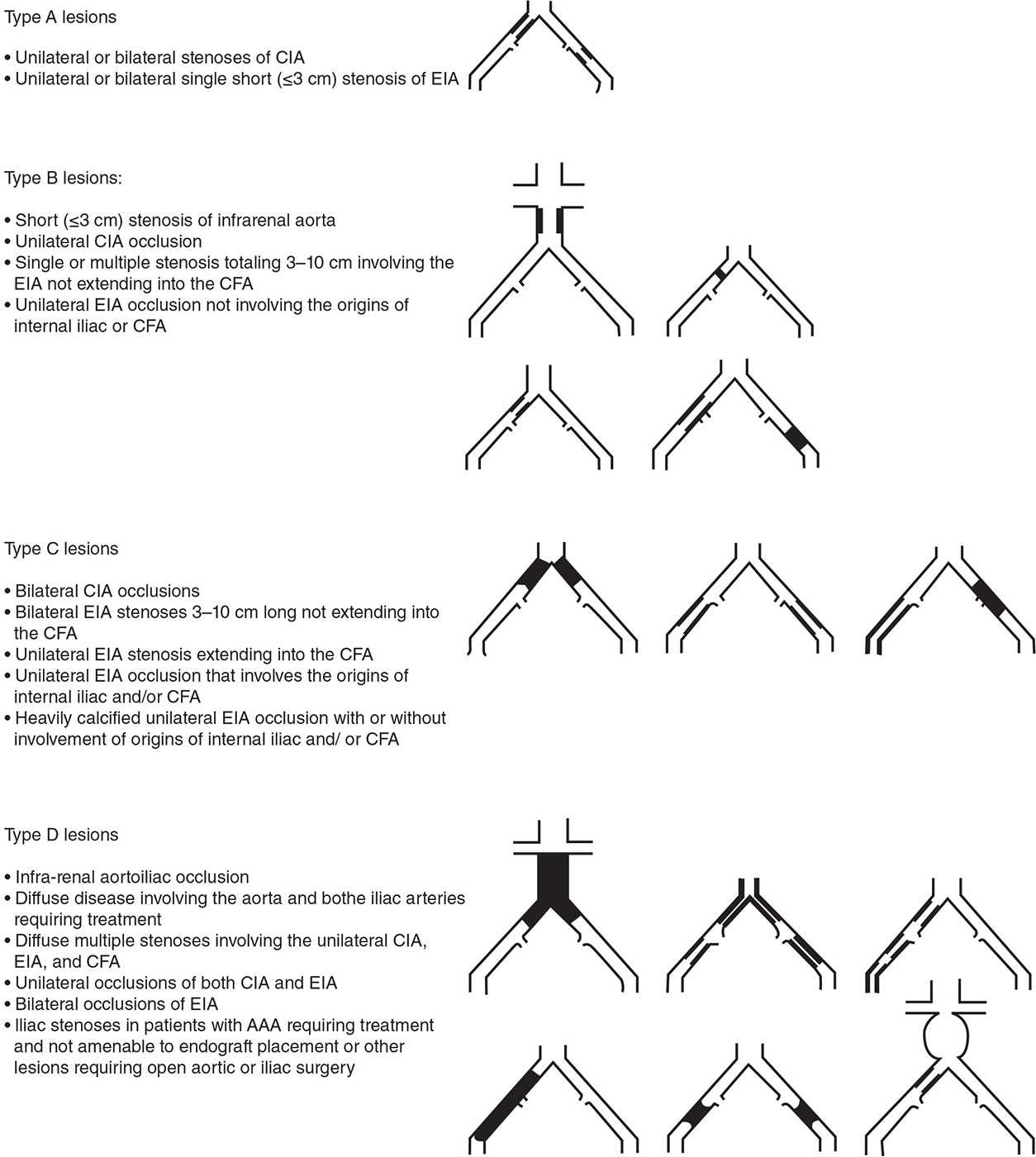
FIGURE 22-10 TASC classification of aorto-iliac lesions. (From Norgren L, Hiatt WR, Dormandy JA, et al.; TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45 (suppl S):S5–S67.)
The efficacy and durability of endovascular techniques when compared directly against open surgical procedures for aorto-iliac PAD have been confirmed in the literature. An earlier study by Holm et al.54 randomized 102 patients to PTA versus surgery for short-segment iliac disease between 1983 and 1988, showing similar success rates in terms of postprocedural improvement in ABIs at discharge and at 1 year between the two strategies. More recently, a study from the Cleveland Clinic by Kashyap et al.55 compared aorto-bifemoral bypass to endovascular therapy in patients with TASC B–D lesions, with over 75% of patients having type C or D lesions. While the primary patency rate at 3 years was higher in the surgical than in the endovascular group (93% vs 74%, respectively), the secondary patency rates were equivalent at 93%. There were no significant differences in safety when comparing the two groups, in terms of perioperative mortality or postoperative complications. Unexpectedly, female gender and smoking were not related to primary patency, whereas the presence of diabetes was related to patency loss. Interestingly, they found a high primary patency rate of 90% for type D lesions treated via an endovascular route.
More data have emerged addressing the feasibility and durability of endovascular techniques in advanced TASC groups, challenging the current AHA/ACC guideline recommendation of deferring such lesions to surgical repair only. Ichihashi et al.56 reported their experience with 533 aorto-iliac lesions, stratified by lower versus higher TASC classification. They found no difference in technical success rates between TASC A and B lesions versus TASC C and D lesions (both were 99%). The TASC C and D lesions were characterized by significantly longer procedural times and higher complication rates (9% for TASC C and D vs 3% for TASC A and B). There were no significant differences in the 1-, 3-, 5-, and 10-year primary patency rates between the groups. Leville et al.57 presented their experience with 92 aorto-iliac occlusions, of which three-quarters were TASC C and D lesions. The overall technical success was 91%, with successful recanalization and stenting of 86% of TASC D lesions. The primary and secondary patency rates were 76% and 90%, respectively, at 3 years, and these did not vary significantly by TASC stratification. An older series by Scheinert et al.58
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


