Carotid Disease
Carotid atherosclerotic disease is an important cause of ischemic stroke and transient ischemic attack (TIA). Diagnosis is made using ultrasound, computed tomography, magnetic resonance angiography (MRA), or angiography. Treatment consists of medical therapy to address the risk factors responsible for the formation and progression of carotid atherosclerosis, and carotid endarterectomy (CEA) or carotid artery stenting (CAS) in appropriately selected patients.
EPIDEMIOLOGY
Approximately 800,000 people in the United States experience a stroke each year, with a 30-day mortality of 10%.1–3 Up to 500,000 more individuals suffer a TIA annually.1 The male-to-female ratio for strokes is greater at ages <65 years (1.50) and less at ages >75 years (0.76); overall, approximately 55,000 more women than men have a stroke each year. With respect to race, blacks have an almost twofold higher risk of first stroke than whites.
PATHOPHYSIOLOGY OF CAROTID ARTERY DISEASE
An estimated 87% of strokes are ischemic in nature, about 15% to 20% of which are attributed to complications of carotid atherosclerotic disease.1,4,5 Carotid stenosis can lead to ischemia via progressive narrowing and impairment of blood supply, or more commonly by providing a foundation for the formation of local plaque rupture and athero-thrombosis that embolizes distally. It is important to note that the severity of stenosis is predictive of ipsilateral stroke risk, with a 5-year risk of 7.8% in asymptomatic patients with <50% stenosis and 18.5% in patients with 75% to 94% stenosis.6 In patients with an occluded artery, the risk is abrogated compared with severe stenosis, with an ipsilateral stroke risk of 9.4% at 5 years. Other studies of patients with asymptomatic disease demonstrate the variability in stroke incidence. In the Veterans Affairs Cooperative Study (VACS) of patients with asymptomatic stenosis >50%, the risk of ipsilateral stroke at 4 years was 10.3% in those treated with medical therapy.7 Similar findings were noted by the asymptomatic carotid artery stenosis (ACAS) investigators who found a risk of ipsilateral stroke of 6.2% at 2.7 years of follow-up in patients with >60% stenosis.7 The risk of stroke provided above is indicative of older studies with medical therapy that predates the current era. In more contemporary studies with optimal medical therapy, the risk of ipsilateral stroke has been shown to be as low as 0.34% per year with a risk of ipsilateral TIA of 1.78% per year.8
Risk factors for carotid stenosis are similar to those for coronary artery disease (CAD), reflected in the fact that more than one-fifth of patients undergoing coronary artery bypass grafting (CABG) have >50% carotid stenosis.9 Conversely, in patients with cerebral infarction, more than 50% have some degree of coronary stenosis, and more than one-fourth have coronary stenoses >50%.10 Material extracted from embolic protection devices (EPDs) after CAS reveal lipid vacuoles, foam cells, fibrin, and platelets, similar to that of coronary lesions. Increasing age results in greater risk of stroke or TIA in both men and women as well as across race.1 The relative risk of stroke is doubled among smokers and returns to baseline levels after 5 years of abstinence.11 Hypertension, diabetes mellitus, hyperlipidemia, and the metabolic syndrome all impart an increased risk.12–14
CAROTID AND ARCH ANATOMY
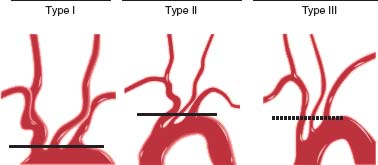
Defining aortic arch anatomy is an important part of carotid and cerebrovascular angiography and is necessary in planning a carotid intervention. The aortic arch is defined as type I, II, or III depending on the relationship of the innominate artery to the inner and outer aortic arch curvature(s) (Fig. 51.1). While the normal arch branching pattern consists of the innominate, left common carotid (LCC), and left subclavian (LSC) arteries (proximal to distal), there are a number of variants. In about one-fourth of patients, the LCC and innominate share a common origin, and in about one-sixth of patients, the LCC originates directly from the innominate artery. Both of these configurations are termed “bovine arch,” though in reality bear no resemblance to the arch of cattle.15 Other variations include an anomalous right subclavian artery that originates directly from the arch distal to the LSC artery, anomalous left vertebral artery originating directly from the arch, and a thyrocervical trunk originating from the aorta.
FIGURE 51.1 Classification of aortic arch type is based upon the relationship of the innominate artery to the inner and outer curvature of the arch. (Reproduced from Krishnaswamy A, Klein JP, Kapadia SR. Clinical cerebrovascular anatomy. Cath Cardiovasc Int. 2010;75:530–539, with permission from John Wiley and Sons.)
The common carotid artery bifurcates into the external and internal carotid arteries (ICAs) at the C3–C4 interspace, and is the most common site of carotid atherosclerosis. The external carotid artery courses anteriorly and supplies numerous important branches to the face and neck, which also serve as collaterals in the setting of severe ICA disease. The ICA is divided into the cervical, petrous, cavernous, and supraclinoid portions, and no branches arise from the cervical or petrous ICA. The supraclinoid portion of the ICA (carotid siphon) bifurcates into the middle cerebral artery (MCA) and anterior cerebral artery (ACA).
CLINICAL PRESENTATION
Carotid disease may be diagnosed incidentally during physical examination or by diagnostic testing, or as part of the workup of stroke or TIA. While carotid auscultation is a common and important part of a comprehensive cardiovascular physical examination, its sensitivity in patients with stenosis >60% is reported to be as low as 56%.16 Therefore, in high-risk patients, carotid ultrasound is recommended for risk stratification. Population screening is not recommended by the United States Preventive Services Task Force (USP-STF), however.17
TIA is defined as symptoms that resolve within 24 hours (most commonly within 30 minutes) and does not have associated acute imaging changes. Carotid-territory TIA or stroke can be reasonably elucidated from a detailed history and physical examination (Table 51.1). Aphasia, dysarthria, or visual symptoms such as ipsilateral amaurosis fugax or contralateral homonymous hemianopia may be present. Sensory and motor symptoms are typically contralateral.
TABLE
51.1 Stroke Syndromes Involving the Major Vascular Territories
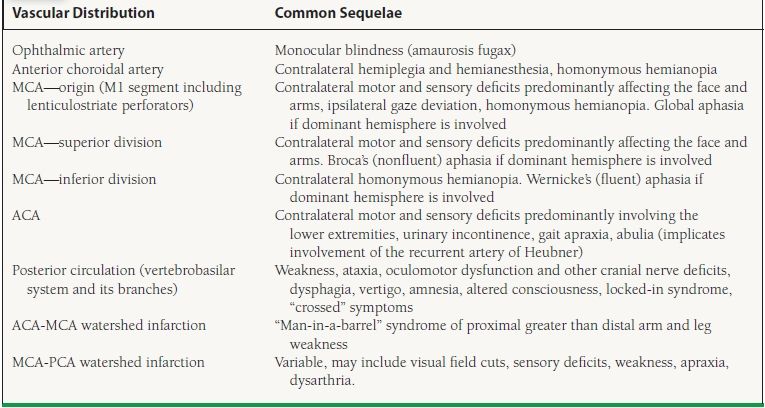
MCA, middle cerebral artery; ACA, anterior cerebral artery; PCA, posterior cerebral artery.
DIAGNOSTIC TESTING
There are various imaging modalities used in the diagnosis of, and procedural planning for, carotid disease.
Carotid Ultrasound
Ultrasonography is the standard noninvasive test for the evaluation of carotid disease. Large studies utilizing carotid ultrasound with angiography as a gold standard have reported sensitivity and specificity of >95% in diagnosing angiographic stenosis >50%.18,19 Standard criteria for the diagnosis of carotid stenosis (the modified Stradness criteria) are listed in Table 51.2. It is important to note, however, that severe left ventricular (LV) dysfunction, severe aortic stenosis (AS), and significant common carotid stenosis can all spuriously decrease carotid velocities and therefore minimize the degree of stenosis calculated.
TABLE
51.2 Doppler Criteria for Diagnosis of Carotid Stenosis
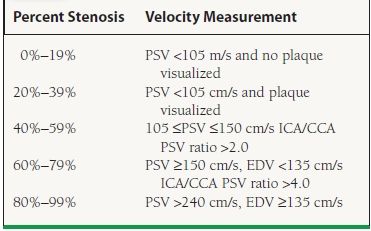
PSV, proximal systolic velocity; ICA, internal carotid artery; CCA, common carotid artery.
Carotid Intima Media Thickness
Carotid intima media thickness (IMT) provides a measure of subclinical atherosclerosis and is usually measured in the common carotid artery. It is a measure of the thickness of the intima and media on two-dimensional (2-D) ultrasound imaging, normally increases with age, and is greater in men than women.20 Carotid IMT may be used for risk stratification purposes in patients without established atherosclerotic disease and intermediate probability as it has been associated with a higher risk of myocardial infarction (MI), stroke, and cardiovascular death.21–23
Computed Tomography Angiography
Computed tomography angiography (CTA) is increasingly used in the evaluation of the coronary and peripheral arteries. In a recent evaluation of CTA, investigators reported a sensitivity and specificity of 77% and 95%, respectively, for diagnosing severe carotid stenosis.24 One significant limitation in the accurate diagnosis of luminal narrowing is the presence of calcium blooming artifact. The diagnosis of moderate stenosis has lower accuracy (sensitivity 67%), similar to MRA in this setting.
Magnetic Resonance Angiography
Carotid imaging using MRA can be performed after gadolinium contrast administration or using time-of-flight (TOF) imaging without contrast. Unfortunately, noncontrast MRA may have poor sensitivity and specificity due to the lengthy time of acquisition (10 minutes), which increases artifact. On the other hand, gadolinium MRA has reasonable sensitivity (95%) and specificity (92%) for diagnosing severe stenosis (>70%).25 Similar to CTA, diagnosis of moderate stenosis is poor (sensitivity 66%).
Angiography
Established as the gold standard for evaluating carotid stenosis, angiography should be performed using digital subtraction angiography (DSA) to “remove” the bones and soft tissues for better visualization of the arteries. Angiography provides highresolution images, allows an analysis of plaque quality (i.e., calcification, ulceration), and enables the operator to evaluate the arch, neck vessels, intracerebral circulation, and collateral filling at the same time. This method is not usually a first-line test given the historically reported risk of transient (1.3%) or permanent (0.6%) neurologic complications.26,27 Notably, a more contemporary series of cerebrovascular angiography showed a much lower rate of complications, with 0.06% transient neurologic deficits and 0.2% iatrogenic dissection, though this series included no arch aortograms and only a small percentage of patients with ischemic cerebrovascular disease.28
MEDICAL TREATMENT
Primary and secondary prevention of carotid atherosclerosis includes risk factor modification and management. The use of CAS or CEA may be relevant in both situations, and are discussed further below.
Antiplatelet Therapy
Historically, aspirin is the cornerstone of antiplatelet therapy. In the Antithrombotic Trialist Collaboration’s meta-analysis of 287 randomized trials enrolling >200,000 patients, they demonstrated a 25% reduction in nonfatal stroke and a 30% reduction in fatal or nonfatal ischemic stroke.29 Additionally, low-doses of aspirin (75 to 150 mg) were just as effective as higher doses, though with the caveat that this conclusion was based on less robust data. It should be noted, though, that the benefit of aspirin has been noted mostly in patients considered to be at high risk. Conversely, patients at low-risk for events have greater potential for harm due to bleeding. As a result, the American College of Cardiology/American Stroke Association (ACC/ASA) guidelines provide a Class I recommendation for aspirin in patients for whom the benefits are likely to outweigh the risks (10-year risk 6% to 10%), though the recommendation is largely based on a reduction in all cardiovascular events and not specifically stroke alone.17 Use of aspirin in women specifically is noted as a Class IIa indication, again for those in whom ischemic stroke risk reduction outweighs the risk of gastrointestinal bleeding or hemorrhagic stroke.17 Recent trials have not convincingly shown that aspirin decreases the risk of first stroke in patients with diabetes (and without CAD), but have produced trends toward benefit. However, professional societies have found it difficult to completely abandon the use of aspirin in this setting. The ACC/ASA guidelines therefore provide a Class IIb recommendation for the use of aspirin in the primary prevention of stroke in diabetics at “high CV risk” with the caveat that the benefits “remain unclear.”17,30,31 All patients with documented carotid disease should receive aspirin (or alternative regimen below).
Clopidogrel is an antiplatelet agent that irreversibly binds the P2Y12 subunit of the ADP receptor, blocking GP IIb/IIIA-mediated platelet aggregation. In the Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial of patients with cardiovascular disease, clopidogrel demonstrated an 8.7% relative risk reduction (RRR) for the primary outcome of stroke, MI, or death.32 There was a trend toward benefit (RRR 7.3%, p = 0.26) in patients with a history of stroke. On the basis of this and other studies, clopidogrel has been given an ACC/ASA Class I recommendation as an alternative agent to aspirin for secondary prevention in patients with a history of TIA or stroke.33
While dual antiplatelet therapy (DAT) with aspirin and clopidogrel is often used in patients after coronary artery stenting, two large studies have not found a benefit in patients with stroke. In both the MATCH (Management of Atherothrombosis with Clopidogrel in High-Risk Patients with Recent Transient Ischaemic Attack or Ischemic Stroke) and CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance) trials, DAT did not significantly decrease stroke outcomes, but did result in a significantly increased bleeding.34,35 The ACC/ASA guidelines therefore provide a Class III recommendation (not recommended) for the use of DAT for secondary prevention of stroke.33
Dipyridamole (DP) is an adenosine deaminase (ADA) and phosphodiesterase (PDE) inhibitor, which results in an increased concentration of cyclic AMP, adenosine, and adenine nucleotides; this inhibits platelet aggregation and causes vasodilation. Large studies using ASA and DP in patients with stroke have found a significant benefit to the use of combination therapy over monotherapy.36,37 Therefore, the
ACC/ASA guidelines provide a Class I recommendation for ASA/DP combination therapy for patients with TIA/stroke, and suggest its use over aspirin monotherapy.33
Anticoagulant Therapy
While anticoagulation with vitamin K antagonists is standard therapy for patients with cardioembolic source of TIA or stroke, there has been no suggestion of benefit over aspirin in patients with noncardioembolic stroke. The Stroke Prevention in Reversible Ischemia Trial (SPIRIT) randomized patients to warfarin (goal international normalized ratio (INR) 3.0 to 4.0) versus aspirin for secondary prevention.38 Patients receiving warfarin had more than double the adverse events, mostly attributed to bleeding complications. The Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) investigators made a similar randomization though with a more conservative INR goal (2.0 to 3.0) but were forced to terminate the trial early due to an almost threefold increase in major hemorrhage.39 Ultimately, due to complications of bleeding and a lack of benefit for patients with atherosclerotic disease, anticoagulation is reserved for patients with a cardioembolic source of stroke or hypercoagulability disorder and is otherwise not indicated for the routine secondary prevention of TIA or stroke.
Antihypertensive Therapy
A large percentage of the US population has hypertension, and numerous studies have documented a 30% to 40% reduction in stroke with blood pressure control.40 While many studies using individual antihypertensive regimens (thiazides, beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, etc.) have shown a reduction in stroke, there is a paucity of data to recommend one specific therapy over another. The treatment of hypertension for both primary and secondary prevention has been given a Class I recommendation by the ACC/ASA.17,33 For primary prevention in patients, treatment to a goal blood pressure (BP) <140/90 mm Hg is recommended, with a goal BP <130/80 in patients with diabetes or renal disease (Class I).17 Secondary prevention guidelines support a goal BP of <120/80 mm Hg with a Class IIa recommendation.33 All of these recommendations are made on the basis of the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7). While no specific agents are recommended for either group due to the small number of trials and limited comparisons, diuretics or the combination of diuretics and angiotensin-converting enzyme inhibitor (ACEi) are provided a Class IIa recommendation (for secondary prevention).33 The use of ACEi or angiotensin receptor blockers (ARBs) is given a Class I recommendation for patients with diabetes.17,33
Antihyperlipidemic Therapy
Statins (3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors) have become the mainstay of antihyperlipidemic treatment for the primary and secondary prevention of cardiovascular events. Studies of statin use in patients with CAD have shown a reduction not only in coronary events but also in the risk of first-time TIA and stroke. In one of the first trials of statin therapy in patients with a history of CAD, the Scandinavian Simvastatin Survival Study (4S), patients had an approximately 30% RRR in stroke or TIA.41 Studies of statin use in patients without established cardiovascular disease have also shown promising reductions in the risk of first stroke. For instance, use of rosuvastatin among a large group of healthy men and women with elevated C-reactive protein in the JUPITER trial provided an almost 50% RRR in stroke.42 The benefits of statin therapy in reducing stroke among patients with and without CAD was also demonstrated in a meta-analysis of >200,000 patients showing an RR of 0.75 in patients with CAD and 0.77 in patients without CAD.43 Ultimately, the use of statin therapy for the primary prevention of ischemic stroke is based upon the National Cholesterol Education Program (NCEP) recommendations (Table 51.3), and is given a Class I recommendation.
TABLE
51.3 NCEP Recommendations for Statin Therapy for Primary Stroke Prevention
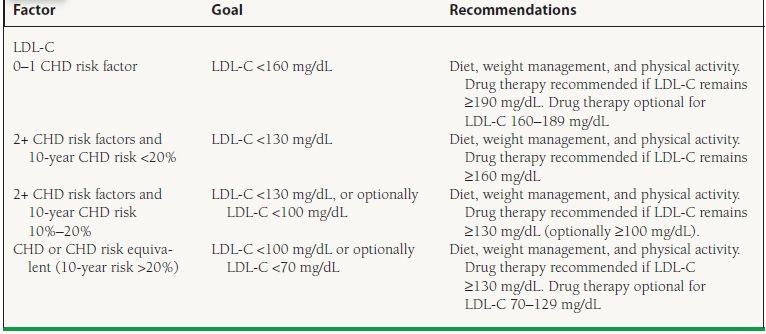
From Goldstein LB, Bushnell CD, Adams RJ, et al. Guidelines for the primary prevention of stroke. A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2011;42(2):517–584, with permission.