(1)
Department of Neuroscience, University of Turin Ospedale Molinette, Turin, Italy
Abstract
Early in the embryogenesis, both primitive proximal ECA and ICA arise separately from the primitive third aortic arch: the ECA from its ventral and the ICA from the dorsal part. The partial involution of the aortic arch, involving its segment distal to the origin of ICA, results in the formation of a common trunk from which develops the common carotid artery (CCA). There are two common carotid arteries. The left common (LC) carotid artery arises from the aortic arch and the right common (RC) carotid artery from the brachiocephalic trunk. The common carotid arteries run cranially in the carotid space, surrounded by the three layers of the deep cervical fascia, called the carotid sheet. Approximately at the level of the hyoid bone, usually between the C4 and C6 vertebral bodies, each common carotid artery divides into the internal carotid artery (ICA) and external carotid artery (ECA).
2.1 Cervical Segment
Early in the embryogenesis, both primitive proximal ECA and ICA arise separately from the primitive third aortic arch: the ECA from its ventral and the ICA from the dorsal part. The partial involution of the aortic arch, on both left and right sides, involving its segment distal to the origin of ICA, results in the formation of a common trunk from which develops on each side the common carotid artery (CCA). In the further evolution, the left CCA is annexed by the developped left fourth aortic arch, and the right CCA from the brachiocephalic trunk (Innominate Artery) proximal remnant of the distally completely regressed rigth fourth aortic arch. The common carotid arteries run cranially in the carotid space, surrounded by the three layers of the deep cervical fascia, called the carotid sheet. Approximately at the level of the hyoid bone, usually between the C4 and C6 vertebral bodies, each common carotid artery divides into the internal carotid artery (ICA) and external carotid artery (ECA).
Cases of a higher bifurcation, up to the first cervical vertebra (Lie 1968), or lower, in the upper thoracic levels (Vitek and Reaves 1973), have been reported. The carotid sheet is a well-defined structure below the carotid bifurcation, though it is incomplete or absent at the level of the oral–nasal pharynx (Harnsberger 1995). The infrahyoid segment of the carotid space contains the common carotid artery and depending on the level of the bifurcation the proximal part of the ICA, the proximal part of ECA, the Internal Jugular Vein (IJV), portions of the cranial nerves IX, X, XI, XII, the Sympatetic Plexus and Lymph nodes. In the infrahyoid segment, the vessels run in the so-called carotid triangle (Som et al. 2003a) (Fig. 2.1) defined by the sternocleidomastoid muscle, laterally and posteriorly, and by the superior belly of the omohyoid and the posterior belly of the digastric muscle inferiorly and superiorly, respectively. In the suprahyoid–infrahyoid segments, the ICA is accompanied by the IJV located posterolaterally, the cranial nerves (IX, X, XI, and XII), the sympathetic plexus, and the chain of lymph nodes.
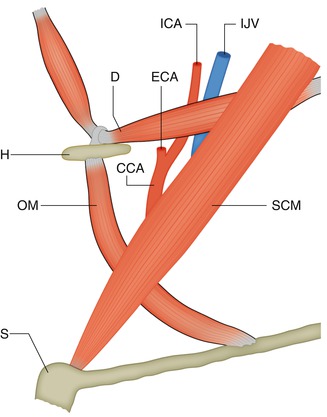

Fig. 2.1
Drawing of the carotid triangle. Lateral-oblique view. SCM sternocleidomastoid muscle, OM superior belly of the omohyoid muscle, D posterior belly of the digastric muscle, H hyoid bone, S sternum, CCA common carotid artery, ECA proximal external carotid artery, ICA infra–supra Hyoid internal carotid artery, IJV internal jugular vein
Near the skull base, the borders of the carotid space (Harnsberger 1995) also called by others (Som and Curtin 2003; Mukherji 2003) the retrostyloid parapharyngeal space can be so outlined: laterally, the parotid space; anteriorly and medially, the parapharyngeal and retropharyngeal spaces, respectively; and posteriorly, the perivertebral space (Fig. 2.2c).
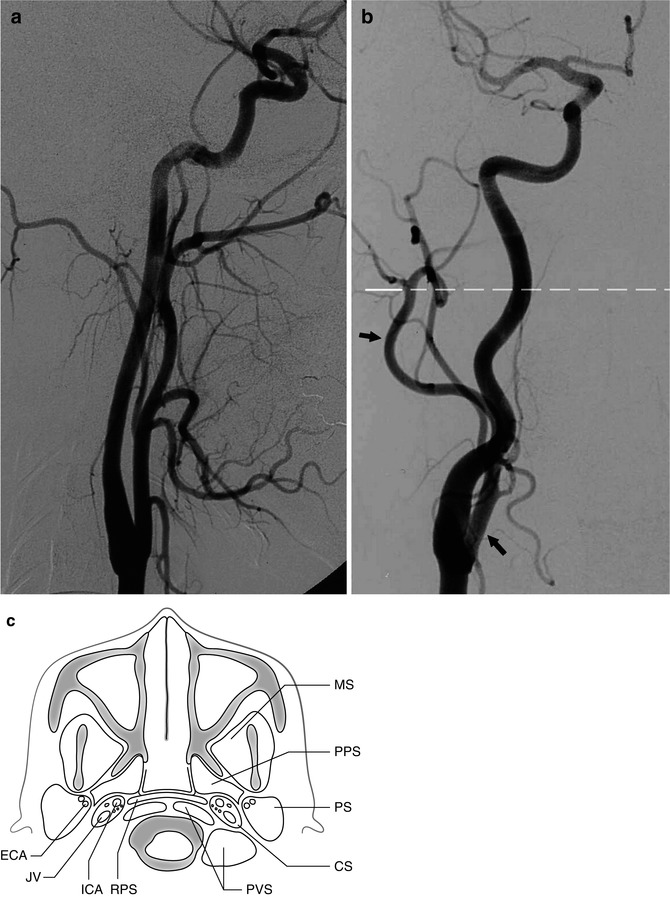

Fig. 2.2
(a) Common carotid angiogram, lateral view, showing the course of the external and internal carotid arteries. (b) Common carotid angiogram, AP view, showing the course of the external carotid artery (ECA, arrow), first medially and more distally lateral to the internal carotid artery (ICA). The dotted line corresponds to the axial plane in (c). (c) Carotid space (CS), surrounded by the parotid space (PS), the parapharyngeal space (PPS), the retropharyngeal space (RPS), and the perivertebral space (PVS). Masticator space (MS). In the carotid space are indicated the ICA (anteriorly) and jugular vein (JV, posteriorly), together with cranial nerves IX, X, X1, and XII. In the parotid space, the ECA runs posteriorly and the retromandibular vein anteriorly. The facial nerve runs laterally
The first segment of the ICA (carotid bulb) is slightly enlarged, becoming smaller and narrower 1–2 cm distally. The bulb can be enlarged, particularly in older, atherosclerotic patients, and tortuosity of the distal segment is frequent in very young and older patients. This tortuosity can be congenital or related to dysplastic or atherosclerotic changes. At its origin, the ICA commonly lies posterior and lateral to the ECA. More distally, it is medial to the ECA (Fig. 2.2a, b) (see Chap. 3).
2.2 Petrous Segment of ICA
The ICA enters the base of the skull at the carotid foramen, anteriorly to the jugular fossa and jugular vein. It runs entirely in the petrous bone, first with a vertical course for about 1 cm, then horizontally medially and slightly upward. Through its course, the ICA lies anteriorly medially and below the tympanic cavity and cochlea. It emerges from the petrous bone, near its apex, running above the cartilage covering the foramen lacerum (Figs. 2.3 and 2.4) and enters the cavernous sinus.
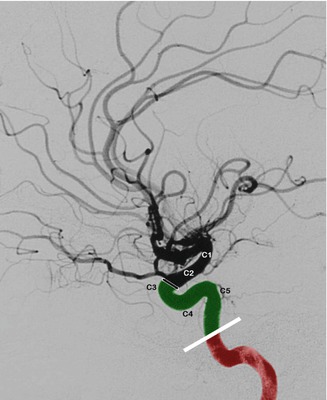
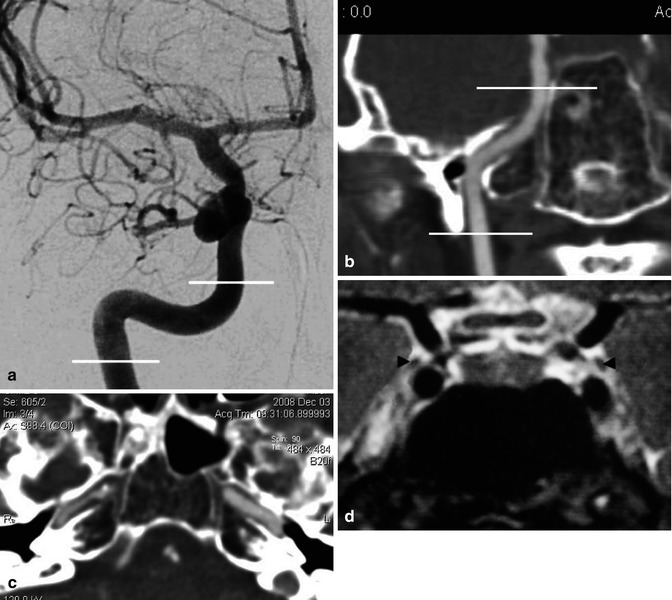

Fig. 2.3
Petrous and cavernous portion of the ICA, lateral carotid angiogram. Petrous portion (in red). Cavernous portion (in green). Dural ring proximal to the origin of the ophthalmic artery. C5, C4, and C3 correspond to the different parts of the cavernous portion of the ICA. C2 and C1 define the supraclinoid and subarachnoid ICA

Fig. 2.4
(a) Carotid angiogram, AP view. The lines define the course of the petrous segment of the ICA, continuing into the cavernous segment. The end of the latter cannot be precisely defined in the AP view. (b) CT angiography, coronal reconstruction, showing the course of the petrous segment. (c) CT angiography, showing the horizontal part of the petrous segment of the ICA running above the foramen lacerum. (d) MRI, coronal view, sellar and parasellar area, showing the course of the ICA in the cavernous sinus. Cranial nerve III (arrowheads)
There are two branches: the caroticotympanic and mandibular arteries. The caroticotympanic artery is an embryonic remnant that supplies the middle ear cavity. There is possible anastomosis with the tympanic branch of the ascending pharyngeal artery (APhA) (see also Sect. 3.4 and Fig. 3.28). The caroticotympanic artery can be involved in tumors of the skull base, particularly in tympanojugular paragangliomas (Fig. 3.24d).
The mandibular artery is an embryonic remnant that usually divides into two branches: one runs in the pterygoid canal, anastomosing with the vidian artery; the other is more inferior, anastomosing with the pterygovaginal artery (see also Sect. 3.7.3 and Fig. 3.27). This artery can be especially involved in the vascularization of angiofibromas (Fig. 3.20). Apart from the above pathological situations, these branches are not commonly visible on the angiogram.
2.3 Cavernous Segment of ICA
This runs in the space formed by the separation of a fold of the dura (Taptas 1982) into two layers: the lateral one is the medial wall of the middle cranial fossa; the other is medial and in close contact in its inferior part with the periosteum of the sphenoid bone (periosteal layer). This space, in which run the ICA, venous channels, and nerves, has been called by Taptas (1982) “the space of the cavernous sinus.” This definition which distinguishes the space from its contents is more appropriate than the commonly used “cavernous sinus” (see also Sect. 9.3.10). In this space, the ICA is directed first forward and upward, then curving posteriorly and slightly medially to the anterior clinoid process. In its course, laterally to the sella turcica and pituitary gland from which is separated by the medial layer of the dura, the artery is surrounded by a venous plexus, and it has a close relationship with cranial nerves III, IV, and VI and the first and second branch of the trigeminal nerves. The nerves run close to the lateral wall, attached to it by dural sheaths. The latter can be connected, forming a thin, irregular inner layer adjacent to the external layer of the lateral wall (Umansky and Nathan 1982). Unlike the other nerves, cranial nerve VI runs inside the cavernous space.
Due to its S-shaped course, the cavernous segment is also called the siphon, which schematically can be subdivided into three segments. The segment called C5 is directed upward, the C4 is horizontal, and the C3 is a posteriorly directed curve up to the dural ring, through which the ICA passes, entering the subarachnoid space (Figs. 2.3 and 2.4). There are two branches of the cavernous segment: one is the meningohypophyseal trunk (MHT), the other is the inferolateral trunk (ILT).
The MHT arises from the medial surface of the C5 segment of the ICA. It gives off a branch supplying the neurohypophysis (inferior hypophyseal artery), which is recognizable on an angiogram as a slight blush. It also gives off dural branches for the clivus and tentorium (clival and tentorial branches). The tentorial branch has been called the artery of Bernasconi and Cassinari (1957), who first reported its angiographic visualization. These dural branches anastomose with meningeal branches of the contralateral ICA and inferiorly with clival branches of the APhA. There are also possible anastomoses with branches of the middle meningeal artery.
The ILT arise from the lateral surface of the C4 segment; it supplies cranial nerves III, IV, and VI and partially the ganglion Gasseri. It gives off dural branches for the dura of the cavernous sinus and adjacent area. In the supply of this area, there is a balance between the ICA system, represented by the ILT, and branches of the ECA, represented by the middle meningeal artery, accessory meningeal artery, artery of the foramen rotundum, and recurrent meningeal artery of the ophthalmic artery. One system can be dominant over the other. Anastomoses are frequently present.
The ILT and MHT are very fine branches (Fig. 2.5), not always recognizable on a lateral angiogram. They can be dilated and well visible when involved in the supply of pathological processes, especially meningiomas and dural arteriovenous fistulas (Figs. 3.25b, 13.7, 13.10, and 13.11).
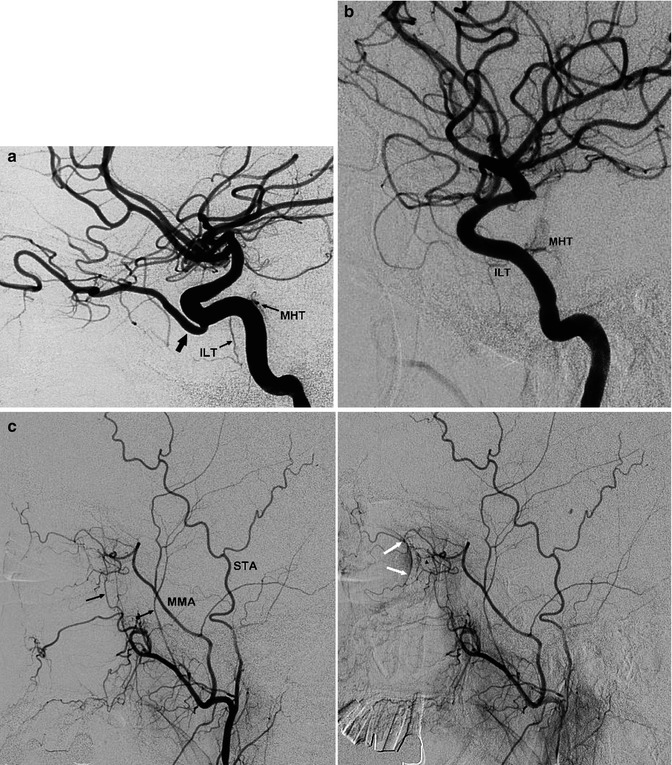


Fig. 2.5
(a) Carotid angiogram. Lateral-oblique view. Origin of the ophthalmic artery from the cavernous portion of the ICA (large arrow). Meningohypophyseal trunk (MHT) and inferolateral trunk (ILT). (b) ICA angiogram, lateral view. There is no ophthalmic artery. MHT, ILT. (c) ECA angiogram, lateral view of the same patient in (b). Origin of the ophthalmic artery from the middle meningeal artery (MMA). There is also a possible supply from the anterior deep temporal artery (arrow). Middle deep temporal artery (arrow with dot). Superficial temporal artery (STA). In the later phase, the ocular complex (arrowhead) and blush of the choroid plexus (white arrow) are recognizable. (d) Different patient: origin of the MMA from the ophthalmic artery. Carotid angiogram, lateral view: ophthalmic artery (O). Lacrimal artery (arrowhead), from which arise the frontoparietal and temporal branches of the MMA (arrows). AP view, ophthalmic artery (O). Branches of the MMA (bidirectional arrow). (Patient with small aneurysm at the level of the posterior communicating artery)
2.4 Supraclinoid Segment of ICA
This begins where the artery goes through the dura and enters the subarachnoid space, running posteriorly, superiorly, and slightly laterally between the anterior clinoid process laterally and the optic nerve medially. The dural ring surrounding the ICA, where the artery enters the subarachnoid space, is closely adherent to the artery laterally, but it is frequently less adherent medially, forming a thin cavity (carotid cave). Aneurysms arising below the dural ring (intracavernous aneurysms) can, however, expand the cave and extend superiorly into the subarachnoid space (cave aneurysms) (Kobayashi et al. 1995; Rhoton 2002).
At the level of the anterior perforated space (APS), the artery divides into the anterior and middle cerebral arteries. The supraclinoid segment can be subdivided into a proximal and distal part, termed C2 and C1. From the origin of its branches, the supraclinoid segment can be more precisely subdivided as follows (Gibo et al. 1981a, 1981b): the ophthalmic segment, from the origin of the ophthalmic artery to the origin of the posterior communicating artery (PcomA); the communicating segment, from the origin of the PcomA to the origin of the choroidal artery; and the choroidal segment, from origin of the anterior choroidal artery to the terminal bifurcation of the ICA.
2.4.1 In the Ophthalmic Segment Arise the Ophthalmic Artery and Superior Hypophyseal Arteries
2.4.1.1 The Ophthalmic Artery
The ophthalmic artery (OA) arises on the superior-medial surface of the ICA, commonly very close to the point where the ICA perforates the dura. It runs below the optic nerve (Hayreh and Dass 1962a, b; Hayreh 1962) and enters, together with the nerve, the orbita through the optic canal. Initially, the artery runs inferolaterally to the optic nerve (first segment), then crosses the nerve forming a bend below or above the nerve (second segment), and runs further medially and parallel to it (third segment). It gives off three types of branches: ocular, orbital, and extraorbital.
The ocular branches include the central retinal artery and the ciliary arteries supplying partially the optic nerve and the ocular bulb. These are the first branches arising where the artery crosses the nerve.
The orbital branches include the lacrimal artery, which supplies the lacrimal gland and conjunctiva. An important branch, sometimes present, is the recurrent meningeal artery, which runs backward and passes through the superior orbital fissure, anastomosing with branches of the middle meningeal artery (MMA). It can be involved in the vascularization of basal meningiomas (Bradac et al. 1990; Fig. 3.25), in dural arteriovenous fistulae (Fig. 13.10), and in the supply of angiofibromas and chemodectomas extending toward the orbita and parasellar region (Fig. 3.20).
Anastomosis of the lacrimal artery with the anterior deep temporal artery can be an important collateral circulation via the OA in occlusion of the ICA (Fig. 3.12). Other branches are the muscular arteries, which supply the muscle and orbital periosteum.
The extraorbital branches are numerous. They include the posterior and anterior ethmoidal arteries. The posterior arise from the first segment, the anterior from the third. These branches have an ascending course and pass through the lamina cribrosa, supplying the dura of the basal anterior cranial fossa. The anterior falx artery arises from the anterior ethmoidal artery and supplies the falx, anastomosing with the falx branches of the MMA. There are anastomoses between the ethmoidal arteries and the internal maxillary artery (IMA) through its sphenopalatine branches. From the latter arise small vessels with an ascending course; they anastomose with the corresponding descending branches that arise from the ethmoidal arteries. These arteries are typically involved in the vascularization of meningiomas of the anterior cranial fossa (Bradac et al. 1990, Fig. 3.25) and in dural arteriovenous fistulas (Figs. 13.8 and 13.15). Involvement in the supply of angiofibromas extending toward the orbita can also occur (Fig. 3.20).
Other arteries of this group are the supraorbital (frequently the most prominent), the dorsonasal, the medial palpebral, and the supratrochlear. These branches anastomose with branches of the ECA, in particular with the facial artery, infraorbital branch of the IMA, and frontal branches of the superficial temporal artery. Such anastomoses may be collateral via the OA toward the ICA when the latter is occluded (Fig. 3.12). Furthermore, these branches can be involved in vascular malformations of the craniofacial area (Fig. 3.16).
On an angiogram (Vignaud et al. 1972; Huber 1979; Morris 1997; Osborn 1999), the OA is always visible; it is better defined in the lateral view. From its origin, it runs superiorly for 1–2 mm, then anteriorly, forming a slight curve with inferior convexity. About 2 cm from its origin, the OA curves abruptly and crosses the optic nerve. Among its branches, the central retinal and ciliary arteries are sometimes recognizable, arising at the level of the above-described curve (Fig. 2.6). Thus, in embolization procedures involving the OA, the microcatheter should be advanced distally to the above-described curve. The blush corresponding to the plexus of the ocular choroid is always visible as a crescent-shaped structure. The ethmoidal arteries are occasionally evident, especially in the lateral view. The anterior falx artery is also easily identifiable, when present, on a lateral angiogram. These arteries can be well developed if involved in pathological processes (Figs. 3.25, 13.8, and 13.15). The other branches are difficult to recognize under normal conditions.
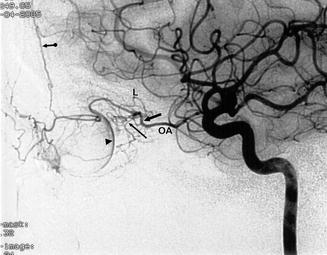

Fig. 2.6
Lateral ICA angiogram. Ophthalmic artery (OA). Bend of the artery around the optic nerve (large arrow). In this area arises the ocular complex comprising the retina and cilial arteries (small arrow). Choroid plexus (arrowhead), lachrymal artery (L), anterior falx artery (arrow with dot)
To explain some variants of the OA, it is useful to recall the most important aspects of its embryogenesis (Hayreh and Dass 1962a, b; Hayreh 1962; Lasjaunias et al. 2001). The definitive OA develops from three sources: the primitive dorsal OA, arising in the intracavernous portion of the ICA and entering the orbita through the superior orbital fissure; the primitive ventral OA, arising from the anterior cerebral artery and entering the orbita through the optic canal; and the stapedial artery (StA), which gives off an orbital branch entering the orbita through the superior orbital fissure. Inside the orbita and around the optic nerve, an arterial anastomotic circle is formed among these three arteries. In the further evolution, the proximal segment of the primitive ventral OA disappears, arising now from the supracavernous portion of the ICA. This artery will become the definitive OA. The primitive dorsal OA regresses, and the intraorbital branches of the StA are annexed by the definitive OA. In this process, important changes can involve the StA, some details of which are presented here.
The StA is the main branch of the hyoid artery, embryonic vessel, arising from a segment of ICA which in this stage of the evolution is very small and incompletely developed. Later, this segment will become the petrous ICA. The StA enters the middle cranial cavity, passing through the tympanic cavity and dividing into intracranial and extracranial branches (Moret et al. 1977; Lasjaunias et al. 2001). The intracranial branch (supraorbital artery) is anteriorly directed, supplies the dura of the middle cranial fossa, and extends into the orbita, with a medial and lateral (lacrimal) branch. These branches enter the orbita through the superior orbital fissure. In some cases, the lacrimal artery penetrates as an isolated branch through the foramen of Hyrtl, located in the greater wing of the sphenoid bone. The second branch (maxillomandibular artery) is directed extracranially and passes through the foramen spinosum, anastomosing with the ventral pharyngeal artery embryonic vessel representing the proximal external carotid artery. From this connection develops the final internal maxillary artery (IMA) and the middle meningeal artery (MMA). The StA disappears, but in some cases, its first segment can persist as a small artery (caroticotympanic branch of the ICA). The extracranial segment becomes the MMA, arising from the developed final IMA; the intracranial segment in the middle cranial fossa partially regresses and is partly annexed by the MMA. The blood flow is now reversed, being intracranial directed. The intraorbital segment is annexed by the OA.
The embryological evolution can vary and lead to a series of conditions with different angiographic patterns (McLennan et al. 1974; Moret et al. 1977; Rodesch et al. 1991b; Morris 1997; Lasjaunias et al. 2001; Perrini et al. 2007). We describe here the most frequent.
The proximal part of the primitive ventral OA does not regress and so the OA arises from the anterior cerebral artery (Hassler et al. 1989). This evolution could also explain the origin of the OA from the distal ICA bifurcation as reported by some authors (Parlato et al. 2011).
The primitive ventral OA disappears instead of the primitive dorsal OA, leading to an intracavernous origin of the OA (Figs. 2.5 and 4.10c, d).
The proximal part of the OA disappears, though the intraorbital section of the StA remains and is connected at the level of the superior orbital fissure with the MMA. In such cases, the OA is only visible on the ECA, not ICA, angiogram (Figs. 2.5b, c).
The lacrimal branch can persist as an isolated branch of the MMA (meningolacrimal artery), entering the orbita through the foramen of Hyrtl and supplying partially the intraorbital structures, while the ocular and neuronal branches arise from the OA. In such cases, the orbital vascularization is partially visible on the ECA and ICA angiogram. There are commonly no anastomoses between these two systems. In other cases, the MMA gives off a branch, which enters the orbita through the superior orbital fissure and anastomoses with the lacrimal branch of the OA.
Another condition is characterized in addition to the MMA by the presence of the recurrent meningeal artery (Figs. 13.10, 3.20, and 3.25). This is a meningeal branch, arising from the OA in its initial segment or from the lacrimal branch; it runs posteriorly through the superior orbital fissure, supplying the dura in the area of the cavernous sinus and tentorium, where it anastomoses with other branches involved in the supply of this region.
The MMA arises from the OA, and so it is only recognizable on the ICA angiogram. This occurs when the intracranial part of the MMA does not develop; the proximal part of the intraorbital–transsphenoidal segment of the StA does not regress and anastomoses with the lacrimal branch of the OA (Fig. 2.5d).
The MMA originates in the petrous segment of the ICA: this occurs when the first and intracranial segments of the STA do not regress and the extracranial portion of the MMA does not develop. In the skull CT, the foramen spinosum is not present, and the MMA is only visible on the ICA angiogram.
Finally, cases of origin of the ophthalmic artery from the basilar artery have been described (Schumacher and Wakhloo 1994; Sade et al. 2004). It is difficult to explain this very rare anomaly considering the classical description of the embryogenesis of the OA. Similarly difficult to explain is the embryological mechanism responsible for the origin of the MMA from the basilar artery as reported by some authors (Seeger and Hemmer 1976; Shah and Hurst 2007; Kumar and Mishra 2012).
2.4.1.2 The Superior Hypophyseal Artery
The superior hypophyseal artery (SHA) is a group of small branches arising commonly from the posteromedial surface of the ophthalmic segment of the ICA. The SHA supplies the infundibulum, the anterior lobe of the pituitary gland, and partially the optic nerve, chiasma, and floor of the III ventricle. The SHA is not recognizable on a normal angiogram.
2.4.1.3 Supply of the Pituitary Gland
The adenohypophysis is supplied by the superior hypophyseal arteries. These run toward the pituitary stalk, where they connect with a network of capillaries continuing in venules forming the so-called venous portal system, through which flow the releasing and release-inhibiting hormones from the hypothalamus to the adenohypophysis. The neurohypophysis is supplied by the inferior hypophyseal artery which is a branch of the MHT. There are anastomoses between the branches of the superior hypophyseal and inferior hypophyseal arteries and that of the contralateral arteries. Each half of the pituitary gland drains into the corresponding cavernous sinus, which continues into the inferior petrosal sinus.
2.4.2 In the Communicating Segment Arises the PcomA
The PcomA arises from the posterior surface of the ICA. It runs posteriorly and medially to join the posterior cerebral artery (PCA) in a close relationship with cranial nerve III, which is laterally and sometimes medially located (Gibo et al. 1981a). An anomalous origin from the OA has been reported (Bisaria 1984).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


