Fig. 11.1
Alcoholic cardiomyopathy. A 54-year-old male, alcoholic, who died suddenly. (a) Heart of 760 g with globoid appearance. (b) Dilatation of both ventricular chambers (RV chamber: 5 cm, LV chamber: 4.5 cm). Ventricular wall thickness is 5 mm in the RV and 12 mm in the septum and free wall of the LV. (c) Hypertrophic cardiomyocytes and interstitial fibrosis (Masson‘s trichrome)
11.3 Cocaine
Several studies have demonstrated that cocaine consumption has major adverse cardiovascular effects due to its actions on both coronary arteries and myocardium. Furthermore, cocaine is a pro-arrhythmic substance due to its effects on Na+ and K+ channels (Table 11.1).
Table 11.1
Cocaine-related cardiovascular pathology
Coronary arteries |
Acute pathology: |
Coronary artery spasm (mediated by α1-adrenergic receptors) |
Ischaemia, especially when associated with severe atheromatosis |
Chronic pathology: |
Accelerated atherosclerosis and thrombosis |
Intramyocardial small vessel disease |
Myocardium |
Left ventricular hypertrophy due to: |
Elevated systolic blood pressure |
Direct stimulation of the myocardiac α-adrenergic receptors |
Replacement fibrosis secondary to ischaemia due to: |
Vasoconstriction |
Accelerated atherosclerosis |
Arrhythmias due to |
Loss of function of Na+ channels (Brugada-like electrocardiographic pattern) |
Loss of function of HERG K+ channels (Bradycardia, Long QT syndrome (LQTS)) |
These effects, in isolation or in combination, make cocaine consumption a risk factor for sudden cardiac death (Fig. 11.2).
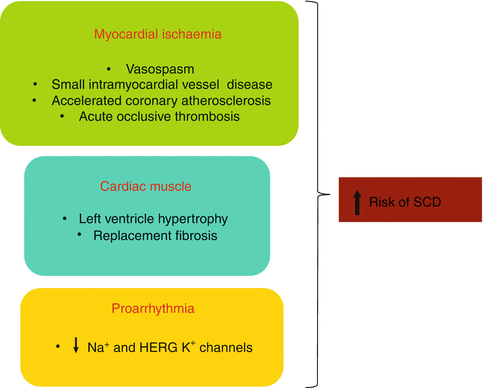

Fig. 11.2
Mechanisms through which cocaine increases the risk of sudden cardiac death (SCD)
11.3.1 Accelerated Atherosclerosis
It has been suggested that repeated administration of cocaine leads to endothelial damage, which favours the development of a premature and accelerated form of atherosclerosis. Lucena et al. (2010) reported severe coronary artery atherosclerosis in 76.2 % of the autopsies performed in cases of SCD associated with cocaine abuse, and atherosclerosis was the main cause of death in 28.6 % of the cases (Figs. 11.3 and 11.4). Cocaine has also been associated with intravascular thrombosis in the coronary and peripheral arteries (Figs. 11.5 and 11.6). Evidence suggests that cocaine activates platelet aggregation and stimulates the secretion of thrombogenic substances from the vascular endothelium.
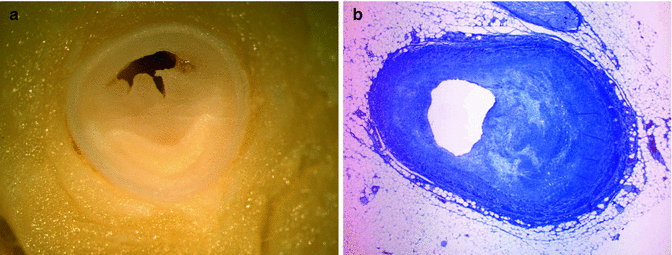
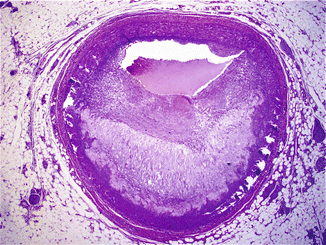
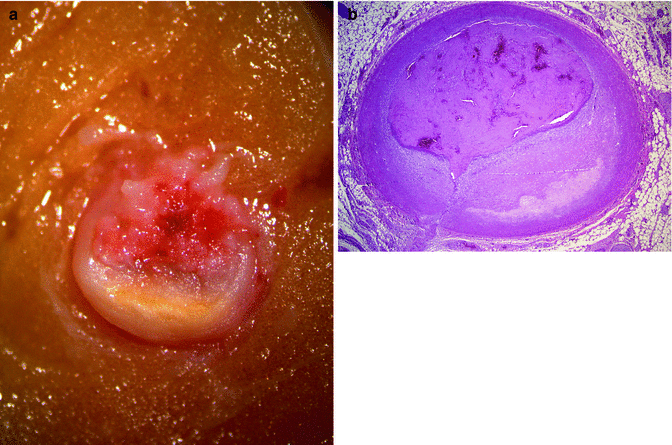
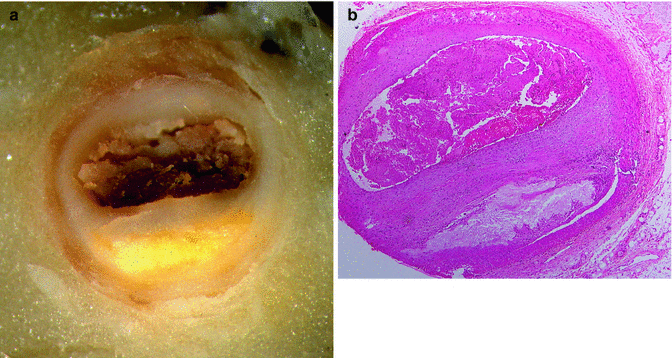

Fig. 11.3
A 21-year-old male, who committed suicide by shooting himself in the head after a weekend of consuming large amounts of cocaine. (a) Severe atherosclerosis in the proximal third of the LAD coronary artery. (b) Microscopic image showing atherosclerosis

Fig. 11.4
A 28-year-old male, with a history of type I diabetes, who suffered cardiac arrest after smoking free-base cocaine. Severe atherosclerosis in the proximal third of the LAD coronary artery, with a large lipid core

Fig. 11.5
A 28-year-old male who died suddenly in a pub. (a) Acute thrombosis superimposed to an atheroma plaque, in the proximal third of the LAD coronary artery. (b) Acute occlusive fibrin platelet thrombosis due to endothelial erosion superimposed to a fibrocellular plaque

Fig. 11.6
A 33-year-old male, with a history of cocaine consumption, who died suddenly at home. (a) Acute thrombosis superimposed to an atheroma plaque, in the proximal portion of the LAD coronary artery. (b) Histological image showing the fibroatheromatous plaque with a small lipid core and acute thrombosis due to plaque erosion
Coronary artery atherosclerosis, with or without added thrombosis, results in acute or chronic myocardial ischaemia (Fig. 11.7).
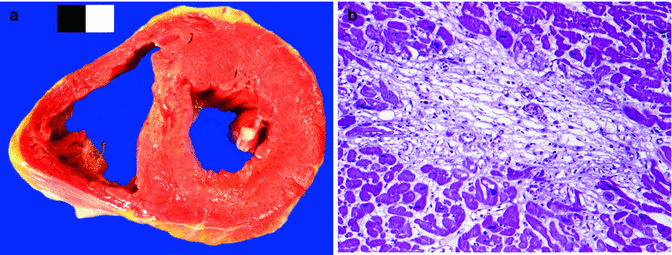

Fig. 11.7
A 28-year-old male, cocaine user, who died suddenly at home. (a) A recent myocardial infarction can be appreciated in the ventricular septum. (b) Microscopic image of the same case showing myocyte necrosis and granulation tissue (about 10–14 days old)
11.3.2 Intramyocardial Small Vessel Disease
In recent years, it has been demonstrated that myocardial perfusion can be diminished, even in the absence of atherosclerosis, in the epicardial coronary arteries. This could be explained by a thickening of the medial layer affecting small intramyocardial vessels that appears in cocaine consumers. This finding is considered a small vessel disease characterised by fibromuscular hypertrophy of the arterioles with medial hyperplasia and endothelial oedema (Fig. 11.8). Hypertrophy in the medial layer of the intramyocardial small vessel could result from sustained vasoconstriction due to the chronic effect of cocaine.
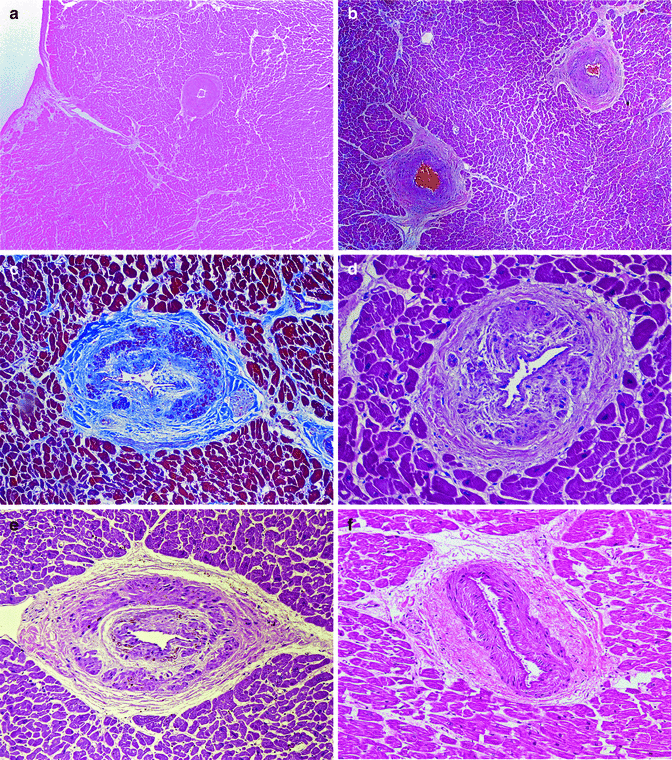

Fig. 11.8
Intramyocardial small vessel disease in cocaine consumers, from 19 to 34 years, who died suddenly. (a–f) Significant periadventitial fibrosis and hyperplasia of the medial layer can be appreciated, which notably reduces the vascular lumen (Masson’s trichrome).
11.3.3 Cardiomegaly with Left Ventricular Hypertrophy
Cardiac hypertrophy per se increases the risk of SCD, even in the absence of cocaine consumption. Increased heart weight is a demonstrated consequence of chronic cocaine consumption, which can in turn favour the development of myocardial ischaemia (Fig. 11.9). Contradictory data exist in the literature regarding the heart weight in cocaine addicts. Some studies report an increase in heart weight whereas other studies show no differences. The most likely cause of LV hypertrophy could be the transient elevation of blood pressure that occurs after cocaine consumption. Another possible mechanism could be a direct stimulation of cardiac α-adrenergic receptors by cocaine.
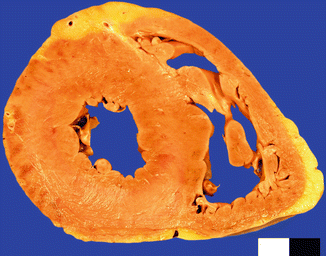

Fig. 11.9
A 26-year-old male who died whilst being arrested by the police as a consequence of excited delirium after cocaine and alcohol abuse. Cardiomegaly (470 g) with concentric LV hypertrophy (20 mm)
11.3.4 Cocaine-Related Myocardial Fibrosis
The high prevalence of myocardial fibrosis encountered in individuals whose deaths are related with cocaine and amphetamine use could highlight the existence of previous ischaemic episodes. However, the healing processes that take place following recurrent contraction band necrosis could also explain the anatomical distribution of the areas of fibrosis reported in cocaine consumers. Underlying silent ischaemia, or myocardial fibrosis as a substrate for re-entry arrhythmias, or both, can increase the risk of arrhythmias. In cases of cardiac hypertrophy secondary to pressure overload, there is also progressive accumulation of collagen fibres in the interstitium, particularly in the LV (Fig. 11.10).
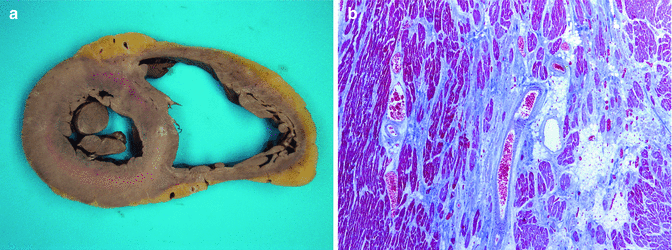

Fig. 11.10
A 31-year-old male, with a history of cocaine use, who died suddenly whilst playing football. Toxicology in blood and urine was negative. (a) Heart of 440 g with an area of septal fibrosis caused by an old myocardial ischaemia. Marked dilatation of the RV. (b) Replacement fibrosis secondary to chronic ischaemia (Masson’s trichrome)
Myocardial fibrosis, either ischaemic or non-ischaemic, has been demonstrated by cardiac MRI in a high percentage of asymptomatic cocaine users.
11.3.5 Cocaine-Related Eosinophilic Myocarditis
Eosinophilic myocarditis has been reported in individuals with cocaine in blood, who died from cocaine overdose or homicide. The frequency of myocarditis in these subjects varies substantially, with some works describing eosinophilic myocarditis in around 5 % of autopsy series, while others report this in up to 20 % of cases (Fig. 11.11).
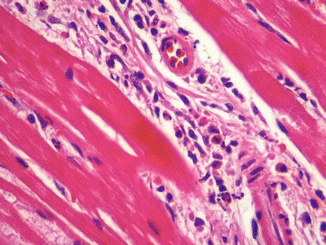

Fig. 11.11
A 26-year-old male who died suddenly after cocaine and alcohol intake. An interstitial inflammatory infiltrate consisting mainly of eosinophils was present in the myocardium
11.4 Synthetic Amphetamines
As a general rule, the cardiovascular effects caused by this heterogeneous group of compounds are quite similar to those of cocaine, with some particularities. Pharmacological actions of these agents are the following:
Increasing the release and decreasing the reuptake of noradrenaline.
Inhibiting monoamine oxidase (MAO).
They are serotonergic agonists.
Increasing the release of vasopressin.
Based on these mechanisms of action, amphetamine and its derivatives (methylenedioxy-methamphetamine –MDMA- or “ecstasy” and methylenedioxyethylamphetamine -MDEA- or “eve”) cause an identical type of cardiotoxicity as cocaine, with increased heart weight, areas of fibrosis and contraction band necrosis. There is also a greater predisposition to suffer accelerated atherosclerosis and intramyocardial small vessel disease, with elevated catecholamine levels as the underlying mechanism (Fig. 11.12).
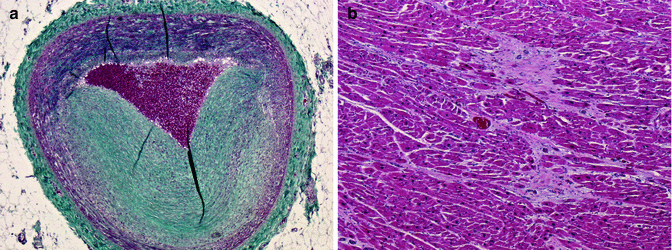

Fig. 11.12
A 39-year-old male who died suddenly. Left ventricular hypertrophy and ischaemic cardiac disease with severe two-vessel (LAD and RCA) coronary disease (a) Severe atherosclerosis in LAD coronary artery (Masson’s trichrome). (b) Large areas of replacement fibrosis in the anterior wall of the LV. Toxicology revealed the presence of amphetamines in blood, urine and vitreous humour
Methamphetamines have multiple effects on the cardiovascular system, with myocardial infarction, aortic dissection and cardiomyopathy emerging as the most important. The histology of methamphetamine-induced cardiomyopathy is similar to that described in laboratory animals: myocardial remodelling that manifests as concentric LV hypertrophy, atypical nuclei, interstitial and perivascular fibrosis, vacuolization of myocytes and hypertrophy in the medial layer of the intramyocardial small vessels. According to Karch (2011), cardiomyocyte hypertrophy, vacuolization and nuclear atypia constitute the diagnostic criteria in cases of death from cardiac arrest related to methamphetamine abuse.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


