(BP stands for blood pressure). MAP is a more accurate index of perfusion in nearly all body tissues except for the coronary perfusion bed in which the diastolic blood pressure is a more important index. However, in cardiac patients undergoing cardiopulmonary bypass, we usually use MAP to assess the overall tissue perfusion.
Although IBP measurement through the arterial line is the most familiar method, it is usually considered as an indirect indicator of the cardiovascular system, since “IBP measurement” depends on two main variables: cardiac output (CO) and systemic vascular resistance (SVR). However, in critical setting, IBP is more accurate than NIBP: When the patient is hypotensive, the blood pressure values measured with NIBP are usually higher than IBP; however, when the patient is hypertensive, NIBP is lower than IBP.
4.2.1 Common Indications and Contraindications for IBP Measurement and Arterial Line Cannulation
4.2.1.1 Indications
1.
Continuous, real-time blood pressure measurement accompanied with heart rate monitoring, like those patients with “impaired hemodynamic status” due to “depressed function of the left ventricle,” “severe myocardial ischemia,” “coronary ischemic syndromes,” “septic or hypovolemic shock,” or “severe right-sided heart failure syndromes” in which vigorous hemodynamic monitoring (including IBP) is essential.
2.
Repetitive measurement of BP (while NIBP is not appropriate or not available, e.g., in patients with multiple, simultaneous burn or fractures in all extremities or compartment syndrome or in patients with morbid obesity in which NIBP monitoring is not possible).
3.
Need for assessment and evaluation of the BP curve and waveform, including the components of the arterial waveform; in these cases, the waveform components are used for a number of clinical uses including the adjustment of intra-aortic balloon counterpulsation using the dicrotic notch or measuring the cardiac output through the arterial wave contour.
4.
Monitoring the effects of pharmacologic interventions or surgical manipulations (e.g., those clinical states in which extensive blood pressure changes would happen, like administration of inotropic agents, manipulation of the heart, major blood vessels, or cardiopulmonary bypass “CPB”).
5.
Severe and/or considerable blood loss (e.g., in severe trauma with extensive hemorrhage or in surgical operations with massive bleeding).
6.
Induced hypotension (due to the nature of the disease and its related surgical operation).
7.
Frequent blood sampling (e.g., to perform blood gas analysis in acid–base or electrolyte disorders).
4.2.1.2 Contraindications
1.
Local infection.
2.
Proximal obstruction (like coarctation of aorta) which would cause underestimation of blood pressure.
3.
Coagulopathies and abnormalities associated with bleeding tendency; these mandate cannulation from more peripheral sites (relative contraindication).
4.
Peripheral vascular disease like Raynaud’s phenomenon and Buerger’s disease (relative contraindication) which mandates more central arterial cannulation.
4.2.2 Technique and Sites of Measurement (Sites of Cannulation), Their Preferences, and Their Potential Complications
Technique: For arterial cannulation, either a “needle through catheter” or a “Seldinger method” could be used, depending on the experience and familiarity of the clinician with each technique. In each technique, administration of local anesthetics on the arterial site before needle and catheter insertion is mandatory to prevent the severe pain due to arterial puncture. Even at times, in the awake patient, administration of incremental doses of intravenous analgesics or sedatives accompanied with respiratory monitoring (like pulse oximetry) is necessary.
However, in the first technique (needle through catheter), the needle is inserted in the place where the finger of the clinician best touches the artery; then, the needle is inserted; care must be taken not to withdraw the needle before the tip of catheter lumen is entered into the arterial lumen as demonstrated in Fig. 4.1. In the Seldinger method, first, the needle is inserted, then the guidewire is sent into the lumen and the needle withdrawn, and, finally, the catheter is conducted over the guidewire into the arterial lumen.
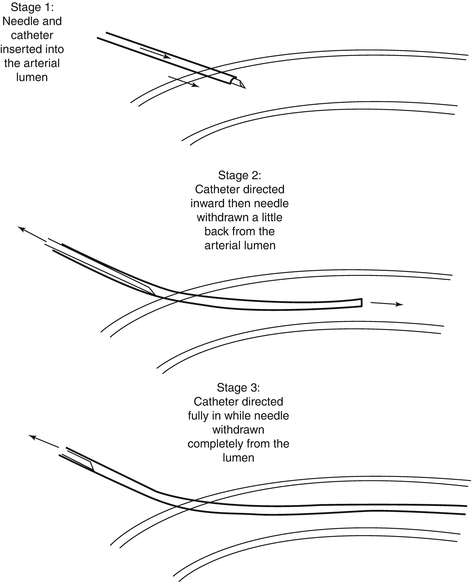
Fig. 4.1
A schematic presentation of needle through catheter technique in three stages; note that care must be taken not to withdraw the needle before the tip of catheter lumen is entered into the arterial lumen (stage 2)
Sites of Cannulation: Ideally, the root of ascending aorta is the ideal site for IBP measurement. However, in nearly all of the cases, this is not a practical approach. So other arteries are used; usually, among all these arteries, radial, femoral, and axillary arteries are the most common sites for arterial line cannulation and IBP monitoring; in other words, radial artery is the 1st, femoral artery is the 2nd, and axillary artery is the 3rd most frequent site for arterial cannulation and IBP monitoring; however, among all of these sites, the radial artery of the nondominant hand (i.e., usually left radial artery) is more common than any other artery used for arterial cannulation.
Other common arteries include brachial, superficial temporal, dorsalis pedis, posterior tibialis, and ulnar artery; none of the above sites are as accurate as the aortic root pressure. In nearly all of these sites, the systolic measurement of BP is more and the diastolic measurement of BP is lower than the aortic root pressure. However, the MAP amount is usually similar in all of these sites.
4.2.2.1 Radial Artery
Using the radial artery for IBP measurement is the most common site among all the others, since it has a good collateral arterial flow. Also, percutaneous puncture method is the preferred method for insertion of the catheter. Regarding its anatomic features, it is superficial to the distal head of radius bone, between flexor carpi radialis and brachioradialis tendons; also, it has a very rich collateral circulation through the ulnar artery and palmar arch. Since it is a peripheral artery, in hemodynamically unstable patients, its cannulation may not be as easy as femoral artery.
Complications of radial artery cannulation:
Bleeding (the most common complication).
Temporary occlusion and temporary spasm of radial artery (with about a 20 % mean frequency in different studies) being a negligible and minor complication; “modified Allen’s test” has not been proved to have predictive value for hand ischemia, though the controversy about this method still exists and some guidelines support doing this test for all the patients.
Permanent occlusion which is a very rare complication.
Other mechanical injuries of the artery rupture (like bleeding, hematoma formation, rupture, and pseudoaneurysm formation).
Discrepancy between radial pressure and the real systolic pressure (especially after some clinical situations like post-CPB period, high-dose administration of vasoactive agents).
Thrombus formation
Sepsis or local infectious complications (cellulitis, abscess, suppurative thromboarteritis).
Local injury to other adjacent tissues (compartment syndrome, paralysis of the median nerve, air embolism, catheter fracture, and carpal tunnel syndrome).
Air embolization to the cerebral arterial system after manual flushing (very rare).
Regional injuries to the hand structures (embolization to the fingers, severe ischemia in hand, or skin necrosis in a region proximal to radial artery).
4.2.2.2 Femoral Artery
The femoral artery is located midway between “anterior superior iliac spine” and “pubic symphysis”; using another anatomic landmark, it is lateral to femoral vein and medial to femoral nerve; it has one of the most accurate arterial waveforms similar to the aortic root (central) arterial wave, regarding both the shape of the wave and the measured values of blood pressure, even in hypotensive and hypovolemic patients. Incidence of complications after femoral artery cannulation is equivalent to radial artery; however, some believe the complications, though infrequent, are more complicated to manage; the complications could be listed as:
1.
Pseudoaneurysm formation.
2.
Bleeding and hematoma formation.
3.
Infectious complications (including sepsis and systemic infection and also local infection); there is possibly no more risk of infectious complications for femoral artery than the upper extremities.
4.
Massive retroperitoneal bleeding (very rare).
5.
Minimal thrombotic risk (due to the large arterial lumen compared with the catheter).
6.
Temporary arterial occlusion (very low incidence).
4.2.2.3 Axillary Artery
The axillary artery provides IBP measurements very near to the aortic root pressure. Its anatomic location is between the triceps and coracobrachialis tendons, in the armpit; i.e., its landmarks are similar to the landmarks used for axillary block. However, performing this procedure is not technically easy for those who are expert. This artery has also extensive collateral arterial flow. Its complications are as follows:
Permanent limb ischemia.
Pseudoaneurysm.
Sepsis.
Paresthesia due to mechanical effects of the needle or the catheter.
Some clinicians have the fear of cerebral air embolism due to retrograde flushing in axillary artery; however, there is no evidence of such complication, and it is a safe approach.
4.2.2.4 Brachial Artery
The brachial artery is subject to inaccuracy, lacks collateral circulation, and is associated with the risk of median nerve injury and compartment syndrome (since it is located in the antecubital fossa); though the occurrence of compartment syndrome is not so much common, it is a real danger, since most of the patients are not fully conscious and also they have been exposed to some anticoagulants. The complications of the brachial artery cannulation are injury
Hematoma
Local infection
Arteriovenous fistula
Pseudoaneurysm
Paresthesia and median nerve injury
4.2.2.5 Ulnar Artery
Due to the location of the ulnar artery, its proximity and attachment to ulnar nerve, and also the greater anatomical perfusion of the distal hand structures from the ulnar artery, some authors have claimed that ulnar artery cannulation might be associated with a higher risk of vascular and organ injury; however, complications of the ulnar artery cannulation are not so much higher than the radial artery, especially if modified Allen’s test is done before arterial puncture and also the radial artery is not injured simultaneously.
4.2.2.6 Dorsalis Pedis
These are the main features of this site:
Excellent collateral flow
Easy cannulation
Minimal patient discomfort
A very low incidence of complications
However, due to the relatively long distance of the artery with the aortic root, BP readings are not accurate with measurements being 5–20 mm Hg higher than the radial artery.
4.2.2.7 Posterior Tibialis
Posterior tibialis artery cannulation is used mainly for the pediatric patients; however, there are not so many complications reported after cannulation of this artery.
4.2.2.8 Superficial Temporal Artery
The superficial temporal artery is one of the end arteries of the carotid; its cannulation has some “serious” complications; due to its disadvantages, this site is not recommended for IBP measurement.
4.2.3 Complications
4.2.3.1 Ischemic Complications
Risk factors for ischemic complications could be classified as female gender:
Preexisting hypertension
Arteritis or other vascular diseases
Size of catheter
Composition of catheter
Time duration for cannulation
Simultaneous use of vasopressors
4.2.3.2 Thrombus Formation
The catheter used for the arterial line could injure the arterial bed and so lead to thrombus formation. There are a number of risk factors which increase the risk of thrombus formation:
The percentage of the artery surface occupied by the catheter lumen; this occupation percentage is calculated according to the following proportion:
External diameter of the catheter/internal diameter of the artery; so for radial artery, a 20 G catheter is recommended.
Teflon catheters are claimed to decrease the risk for “thrombus formation,” though this issue is controversial.
Decreased cardiac output increases the risk of thrombus formation.
Hematoma formation increases the risk for radial artery occlusion and thrombus formation.
Systemic administration of aspirin or low-dose heparin decreases the chance for thrombus formation.
Increased time for catheter preservation increases the risk of thrombus formation (the risk is especially increased after 48–72 h).
4.2.3.3 Infectious Complications
These include sepsis, local infections, and infectious pseudoaneurysm. The main issue in prevention of these complications, devoid of the site of the arterial line, is to strictly adhere to aseptic techniques, for both the arterial puncture site and the related equipments (including the Luer locks and the connecting tubes). Also, any underlying inflammation or infection should be a warning against using the site for arterial blood pressure monitoring.
4.2.4 Technical Considerations in Arterial Line Cannulation
According to the French Society of Anaesthesia and Intensive Care (1995) guideline, the following steps should be followed in arterial line cannulation:
1.
Use Teflon or polyurethane catheters (maximal size of 18 G for femoral or axillary arteries and 20 G for the other arteries).
2.
Maximal length of catheter for small arteries (i.e., radial and dorsalis pedis arteries) is preferred to be 3–5 cm.
3.
Heparin coating of catheters for prevention of complications is not proved.
4.
Using salts for radiopacity is not useful and may even have thrombogenicity.
5.
It is recommended to administer a flush device; the device should have a constant flow of 2 mL/h; at the same time, it is recommended to use a fast flush valve which is connected to normal saline under pressure.
6.
Manual intermittent flushing with a syringe is contraindicated.
7.
Addition of heparin (2,500 IU.500 mL−1 of flush solution) increases the duration of catheter patency and is recommended for catheterizations of more than 24 h duration.
8.
Ready-for-use devices are to be preferred.
9.
Distortion of pressure wave may be minimized by employing low-volume, low-compliance, and low-resistance devices.
10.
The number of connections should be as low as possible and all of Luer-lock type
11.
The stopcocks should be clearly identified to minimize the risk of accidental intra-arterial injection.
12.
The device should be transparent for disclosure of bubbles, which lead to waveform distortion.
13.
For catheter placement, the operator should follow the usual preparation as for any aseptic surgical procedure with cap, mask, gloves, and sterile towel.
14.
The insertion site should be prepped either with chlorhexidine or povidone-iodine.
15.
In the conscious patient, local anesthesia by injection and/or topical application (EMLA) is recommended.
16.
Direct arterial puncture should be preferred rather than transfixion method.
17.
It is recommended to use percutaneous cannulation.
18.
Needle-catheter assembly should be advanced as slowly as possible to prevent arterial transaction; blood return confirms arterial placement.
19.
Resistance against needle advancing is a landmark of error.
20.
Catheterization of deep vessels is facilitated by Seldinger technique, which is recommended whatever the site of placement when long-term monitoring and/or difficulties of insertion are foreseen.
21.
The radial artery is the site of choice for elective cases. The nondominant hand should be preferred. Puncture must be preceded by assessment of adequacy of the collateral flow by the Allen’s test.
22.
The femoral artery is a valuable site for emergency situations. Before catheterization, the artery should be auscultated for a murmur.
23.
Puncture of a vascular prosthesis is contraindicated.
24.
The dressing should be changed every 4 days only.
25.
The dressing is recommended to be visible as much as possible to check for any possible leakage of blood; this issue mandates repeated checking of the catheter and its attachments and also minimal application of dressing material.
26.
Whether peripheral or central arteries are used, the area distal to the puncture site should be checked for any possible signs or symptoms of ischemia, including change in color, temperature, or distal pulse; of course, it may even mandate Doppler assessment of distal flow.
27.
Sites of blood withdrawal should be manipulated with compresses soaked with chlorhexidine or povidone-iodine.
28.
The arterial catheter is only changed in case of evidence of local infection or ischemia.
29.
The catheter removal should be considered as an aseptic surgical procedure, and the catheter completeness has to be checked.
30.
A systematic culture of the catheter is not required.
31.
Also, it has been demonstrated that Doppler ultrasound-guided cannulation may improve the results and decrease the rate of complications.
4.2.5 Arterial Line Transducers and the “Coupling System”
The arterial line transducer and the “coupling system” is the name for the connections from the arterial line catheter connected up to the monitor. Its function is to change the mechanical data into the electrical data used for the monitor display screen. This system contains the following elements:
1.
Transducer: The main “exchange system” which transforms mechanical data to electrical data through a delicate diaphragm usually made of silicon.
2.
Tubing: The mediator between catheter and the transducer.
3.
Flushing System: Prevents clot formation throughout the catheter-transducer assembly by a continuous infusion of saline (usually 1–3 mL/h). This solution was used to be heparin-rinsed; however, recent studies discourage usage of heparin in such solutions for repetitive washing to prevent the real possibility of heparin-induced thrombocytopenia (see Chap. 6 “postoperative coagulation and bleeding” for explanation of heparin-induced thrombocytopenia).
Zeroing: A very important technical consideration is frequent zeroing of the arterial line monitor in order to gain “real” zero readings and then to measure the blood pressure correctly. Each monitor has its recommendations for zeroing based on the manufacturer; however, considering the appropriate level, especially when the patient is in positions other than supine, is an important feature of zeroing. When the patient is in supine position, the level of the transducer should be positioned at the level of the fourth intercostal space which is approximately at the level of midaxillary line; frequent zeroing might increase the accuracy of readings.
Calibration: In order to prevent biased readings, calibration should be done in a timely fashion, especially after each episode of blood sampling, when there is a major change in arterial blood pressure, or whenever the readings are doubtful (Mandel and Dauchot 1977; O’Rourke and Avolio 1980; O’Rourke and Yaginuma 1984; Moran 1990; Clark and Kruse 1992; Cockings et al. 1993; Klepper et al. 1993, Ludbrook et al. 1993, Runciman et al. 1993, Franklin 1995a, b; Horlocker and Bishop 1995; Anderson 1997; Kuhn 2001; O’Rourke et al. 2001; McGhee and Bridges 2002; Scheer et al. 2002; Cousins and O’Donnell 2004; Langesaeter et al. 2008; Nichols et al. 2008; Brzezinski et al. 2009; Wilcox 2009; Augusto et al. 2011; Chee et al. 2011; Ranganath and Hanumanthaiah 2011; Wax et al. 2011).
4.3 Central Venous Pressure Monitoring
Since its first use in 1929 by Werner Forssmann, central venous pressure catheter (CVP catheter or CVC) has gained widespread use all over the world. CVP is the pressure of blood inside the central intrathoracic veins or the pressure of the right atrium. It is usually measured through a central venous catheter (most commonly known as CVP catheter or CVC). CVP is a very good surrogate for right ventricle pressure and is commonly used as an estimate of left ventricular preload; however, its use as an indicator of right ventricle pressure is not always correct, and many do not rely on the absolute CVP measurement as the index for preload due to many different factors affecting the exact CVP measurement. To compensate for this defect, a number of clinicians rely on the trend of its changes for assessment of the preload status of the patients, though this approach has its own “demerits”; nevertheless, CVP has its own many uses and is among the most commonly applicable devices for critical patients including cardiac surgery patients.
CVCs are used frequently not only for assessment of CVP but also for administration of drugs and fluids. Of course, CVCs are not considered as the primary route for urgent and rapid fluid replacement. Instead, peripheral large-bore catheters are considered for rapid fluid administration.
Many different indications have been cited for CVP; however, a number of them are mentioned here, and a detailed list could be found in Table 4.1.
Table 4.1
Indications and contraindications for central venous catheters (CVCs)
Indications |
Administration of pharmaceuticals (esp. the vasoactive or irritant drugs) |
Monitoring the hemodynamic parameters (esp. loading status including CVP) |
Transvenous pacing |
Rapid fluid administration (in trauma or in procedures mandating large fluid shift or blood loss) |
Poor peripheral IV access |
Frequent or rapid aspiration (blood aspiration for frequent venous sampling or rapid air emboli aspiration in specific surgeries) |
Total parenteral nutrition (TPN) |
Continuous renal replacement therapy, temporary hemodialysis, plasmapheresis, or apheresis |
Contraindications |
Absolute contraindications |
SVC syndrome causes CVC to be useless and possibly increases the CVC risks for the patient |
Relative contraindications |
Coagulopathies |
Patients having pacemaker or ICD insertion in the 6 previous weeks |
Anatomic abnormalities (due to coexisting pathologies or recent surgical manipulations disturbing the normal anatomy of the region) |
Carotid disease especially in the presence of contralateral carotid involvement |
4.3.1 Indications for CVC Insertion and Usage
1.
Fluid administration and management (including assessment of loading status, patients with poor peripheral IV access).
2.
Continuous renal replacement therapy and/or temporary hemodialysis (although usual multi-lumen 7 F, 20 cm catheters have a much lower capacity for fluid administration compared with usual peripheral IV access).
3.
Diagnostic measurements (pure values and changing trends of cardiac loading pressures).
4.
Pharmacologic interventions (especially the vasoactive drugs or irritant drugs and plasmapheresis or apheresis).
5.
Other indications (like transvenous pacing).
4.3.2 Central Venous Pressure Curves
The CVP curve is composed of five main waves, namely, a, c, x, v, and y which are the final result of interactions mainly occurring between right atrium (RA), right ventricle (RV), and the tricuspid valve (TV); however, among them a, c, and v are upward deflections, while x and y are downward deflections. These waves mainly demonstrate the right atrial pressure (RAP) as the following order (Fig. 4.2):
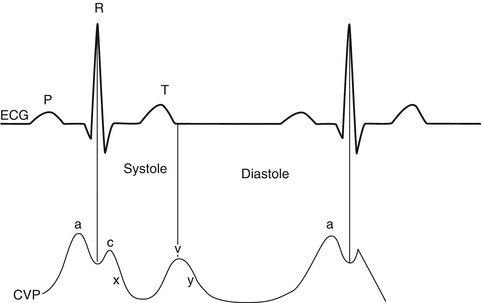

Fig. 4.2
CVP waves and the relation with ECG
a: Atrial contraction causes an increase in RAP; it appears just after P wave in electrocardiography.
c: Isovolemic contraction of RV increases RAP and creates c wave.
x: RV contraction pulls TV away from RA and decreases RAP, so we would have a downward deflection wave.
v: Blood fills RA during late RV systole which increases RAP and again produces an upward deflection.
y: Finally TV opens and causes emptying of RA, so again we see a downward deflection.
4.3.3 Technical Considerations for Insertion of CVC
There are a number of well-recognized approaches for CVC insertion, “internal jugular vein” and “subclavian vein” being the two most common approaches while the right internal jugular vein is more common than the left one, while some believe that left internal jugular approach is as easy as the right side.
4.3.3.1 Right Internal Jugular Vein Approach
The internal jugular vein (IJV) was first described by English in the 1960s and is the most frequently used approach for CVC insertion; it causes less complications, has a straight course to the RA, and has lower chance for thoracic duct and pleural dome puncture, both due to anatomic properties of right IJV, although some have claimed that the left IJV approach is as much easy. It also has a very high success rate (>90 %) even in infants and children accompanied with very low rate of other mechanical complications like arterial puncture, pneumothorax, and hematoma needing surgical intervention compared with all the other methods. Even in anatomically “difficult” patients, the IJV approach has a specific feasibility. The course of IJV especially the right one is straight, short, and with distinct anatomical landmarks; it is without venous valves until superior vena cava and right atrium and could be reached by most anesthesiologist even during the operation time. Its most common and easiest “central approach” described in 1970 by Daily et al. is even the simplest one among all the right IJV access methods in which the needle tip is inserted into the “apex of Sedillot’s triangle, which is between the sternal and clavicular heads of sternocleidomastoid (SCM) and the related portion of the clavicle located between the two SCM muscle heads.” See Fig. 4.3.
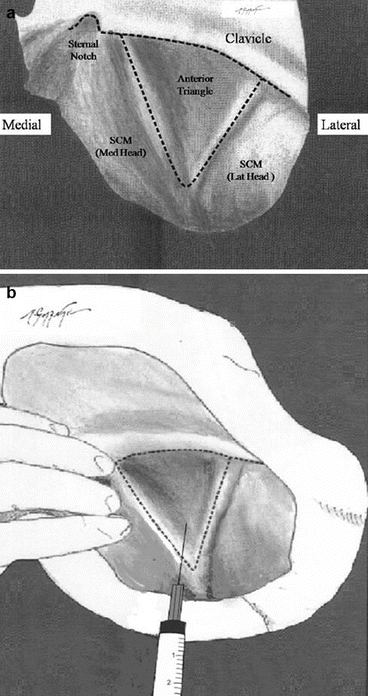

Fig. 4.3
A schematic presentation of the right anterior triangle (Sedillot’s triangle) used for internal jugular vein cannulation when looking from above the patient’s head. (a) The anatomic landmarks of the triangle, SCM sternocleidomastoid muscle, Lat lateral, Med medial. (b) Anatomic location for needle punctures near the apex of triangle towards the ipsilateral nipple
Correct sizing of the catheter length is a very challenging issue.
The following steps should be followed for insertion of CVC through right IJV:
1.
Put the patient in supine position with a little leftward rotation of the head.
2.
Gently extend the neck; excessive extension would distort the “favorable anatomy.”
3.
Check the anatomic landmark of the neck (Sedillot’s triangle) once more before draping.
4.
Use basic monitoring including electrocardiography (ECG), pulse oximetry (SpO2), and noninvasive or invasive blood pressure measurement.
5.
Use a “mild” degree of sedation if the patient is not anesthetized or not sedated accompanied with supplemental oxygen.
6.
Administer strict aseptic techniques including hand washing, using sterile gown and gloves, and sterile preparation from the mastoid to the sternal notch (2 % chlorhexidine is superior to others like 10 % povidone-iodine).
7.
Drape the patient with a large drape.
8.
Put the patient slightly in head-down position except for patients with cardiovascular or respiratory disease.
9.
In awake patients, use 1 % lidocaine solution for local anesthesia using a 25 G needle after another check of landmarks.
10.
Use a 22 G finder needle to find IJV.
11.
Introduce the 18 G needle from the apex of the Sedillot’s triangle from between the two heads of SCM towards the ipsilateral nipple with a 30–45° angle from the skin plane.
12.
If you could not draw dark blood, relocate the needle a bit laterally or medially in a fanwise model; beware of arterial puncture risk.
13.
Check patency of the needle if there is not successful venous puncture yet; also, check the anatomical landmarks once more.
14.
The two venous walls of a central vein are at times compressed against the needle; withdrawing the needle gently usually causes sudden filling of the syringe in such cases.
15.
After appropriate backflow of relatively dark blood, insert the guidewire through the needle; there should not be any resistance in the course of guidewire insertion; otherwise, the guidewire path is not correct; usually the guidewires used for adult CVCs are 0.032–0.035 mm, so the guidewire passage should be never forceful.
16.
The blood flow should be non-pulsatile except for cases of severe tricuspid regurgitation or high right ventricular pressure, in which the backflow of IJV would be pulsatile; if you are doubtful regarding the blood flow to be arterial or venous, attach a sterile stopcock to the 18 G needle, which has an extension tube attached to one of its heads; ask a colleague to attach the other head of the extension tube to the pressure monitoring to rule out potential arterial puncture.
17.
The guidewire should be always in control, regarding both sterility of its distal end and potential arrhythmias of its proximal head; the former needs protection from contact with the unsterile adjacent objects, and the latter mandates careful ECG monitoring; in most adult cases, guidewire advancement below 20 cm prevents unwanted complications.
18.
Withdraw the needle after guidewire installation; use a number 11 scalpel blade before advancing the dilator (especially when a pulmonary artery catheter “PAC” introducer sheath or a large-bore CVC is used); care should be taken not to exert inappropriate force on the catheter; otherwise, guidewire would easily be kinked; on the other hand, excessive dilator advancement would result in unwanted vascular or tissue trauma.
19.
After dilator removal, while the guidewire is still in place, insert the catheter while caring to take the distal end of the guidewire out of the port of the CVC before the catheter is fully inserted; loss of control over the distal part of guidewire could result in catheter embolization, an unwanted complication discussed later.
20.
Using this approach, CVC should be introduced no more than 15–17 cm in adult men and 13–15 cm in adult women to prevent CVC-induced cardiac tamponade (discussed later).
21.
The CVC lines should be de-aired and washed to prevent clotting.
22.
Sterile dressings (without any antibiotics) should be used in place.
23.
Objective confirmation of the CVC tip which is done by CXR after catheter placement; the tip of the catheter should be above the carina which is approximately at the level of T3–T4 thoracic spines, which is also equal to 3rd rib or azygous vein.
4.3.3.2 Left Internal Jugular Approach (LIJV)
The technique is similar to right IJV; however, the dome of the left pleura is higher than the right pleura, and also, thoracic duct passes from the left; these two anatomic features increase the risk for two important complication: pneumothorax and chylothorax. Chylothorax is exclusive to the left hemithorax. The LIJV is shorter than the right IJV, but the venous path from the left side to superior vena cava is longer than the right side. However, the chance for superior vena cava injury in left IJV is higher than right IJV since the path of right IJV is straightforward compared with left IJV.
4.3.3.3 Subclavian Approach
This approach has some specific features, more patient comfort, an easy approach, and lower infection rate and often used for long-term IV therapy but not monitoring purposes.
Method:
1.
Monitor the patient, accompanied with supplemental oxygen and mild sedation.
2.
Head-down the patient.
3.
Rotate the head laterally.
4.
Use a role beneath the two scapulae.
5.
Use local anesthesia in a point just below the clavicle, between the lateral and middle thirds of the clavicle.
6.
The 18 G needle should be passed below the clavicle being directed to the sternal notch.
7.
After appropriate backflow of relatively dark blood, insert the guidewire through the needle.
8.
Other steps are similar to internal jugular approach.
9.
Due to the potential risk of vascular injury or pneumothorax, one should keep in mind that a maximum of three tries from this approach is allowable, and also, bilateral (right and left subclavian) tries are forbidden since bilateral try would be potentially lethal.
4.3.3.4 External Jugular Vein
A simple, really less risky approach, though having lower chance for success; of course, there are a few concerns for this approach:
1.
Never use a dilator for these veins.
2.
90° abduction of the ipsilateral arm increases the success.
3.
The vein course is sometimes tortuous, and the venous path could not be used for introduction of the catheter in nearly 20 % of patients.
4.3.3.5 Femoral Vein
Usually used when IJV or subclavian approaches are not available (e.g., in neck injuries or thoracic involvements). The femoral vein is entered in a place just medial to the femoral artery pulse; however, the needle should be introduced to the vein distal to the inguinal ligament to prevent the risk of retroperitoneal bleeding. Besides, femoral artery or femoral nerve injury, infectious complications, and thromboembolic complications are the main other potential complications of this approach. Two different length catheters have been used in this approach: “40–70 cm” and “15–20 cm” catheters; both are near to the SVC measurement but not exactly the same figures.
4.3.3.6 Peripheral CVC
Peripherally inserted catheters as CVC have been used to decrease complications of CVC; but there is no difference between the two methods regarding infection rate. However, peripheral CVCs have more complications like “catheter tip malpositioning, thrombophlebitis, and catheter dysfunction”; hence, central CVCs seem to be preferred over peripheral catheters.
4.3.4 Ultrasound-Guided CVC Insertion
This method was introduced for the first time in 1984 and could improve success and decrease the rate of complications, so increasing patient safety, especially in IJV approach and in inexperienced hands. Though adult patients are more frequently said to benefit this method, ultrasound-guided CVC insertion has been shown efficacious in pediatric patients. A 7.5–10 MHz probe, covered by a sterile sheath, used by the nondominant hand, finds the transverse (short) axis at first in order to find IJV vein lumen, which is larger, laterally located, and non-pulsatile compared with the medially located carotid artery; the transverse axis is also used for detection of the needle entry to IJV lumen. Then, the longitudinal view (long axis) is used to confirm the appropriate passage of the guidewire into the lumen of IJV. However, in other approaches except for IJV, there is not much great utility for ultrasound-guided CVC insertion because of the sonographic “shadows and distances” between the skin and the vein lumen.
4.3.5 Complications of CVCs
One of the very common topics in everyday practice of cardiac patients is the minor and major complications of CVCs; many complications have been attributed to CVCs, and too many studies have been published in this topic; the majority of these complications are not common; however, a few could potentially lead to major events and even death. These complications are categorized in four main categories and discussed more in the following paragraphs:
1.
Mechanical (vascular injuries, tamponade, nerve injuries, pneumothorax, tissue trauma, etc.)
2.
Thromboembolic
3.
Infection
4.
Other complications
4.3.5.1 Mechanical Complications
These include mainly vascular injuries (arterial puncture, venous injuries), cardiac tamponade, neural injury, tissue trauma, pneumothorax, and catheter or guidewire embolization. Vascular injuries could be arterial or venous.
Arterial puncture is the most frequent among all acute complications, often resulting in local hematoma, which usually resolves after a few minutes of local compression. Few numbers of the patients (especially when major arteries including aorta are injured) need more sophisticated care including emergent consult with a vascular surgeon. If arterial puncture is unintentionally used instead of central vein for catheter placement, the catheter should be removed urgently (except for very unusual circumstances) to prevent unwanted organ embolizations including CNS injuries. However, the risk of arterial bleeding at the puncture site is always potentially serious, needing more vigorous assessments and possible interventions by a vascular surgeon. If a central venous catheter is misplaced anatomically, the location of the catheter tip should be assessed. If it is not in a potentially risky place, we might withdraw the catheter; however, if the catheter tip is located in a risky organ which could not be compressed after catheter removal, or if the course of catheter passes through such an anatomic location, then the catheter should not be removed before an emergent consult with a vascular surgeon.
Other vascular injuries are frequent and diverse including minor venous injuries, hemomediastinum, hydromediastinum, hemothorax, hydrothorax, and chylothorax. And finally, delayed vascular injuries have been reported including different types of fistula between veins, arteries, bronchus, or other adjacent tissues, all being rare but needing vigorous attention and care.
Hemopericardium is the 2nd common and the most lethal complication of CVC placement. It is usually due to perforation of right ventricle, right atrium, or the segment of superior vena cava located inside the pericardium. The rupture would cause sudden cardiac tamponade blood or fluid tamponade. This is a delayed complication of CVC occurring usually in the 1st week after CVC placement and is usually preceded by arrhythmia unresponsive to anti-arrhythmia treatments. Mortality rates more than 80 % are reported for this complication. Would the clinician perform objective documentation of the CVC tip location (i.e., by CXR), this lethal complication could be prevented in many cases.
Pneumothorax is another mechanical complication occurring more commonly after subclavian approach; however, the IJV approach (especially the left IJV) could also lead to pneumothorax though with a lower rate compared with subclavian approach. Minor cases are treated with supportive care, while others need chest tube insertion. Vigorous care should be devoted to high-risk patients especially those underlying mechanical ventilation or those in whom multiple or bilateral punctures have been done to prevent the occurrence of tension pneumothorax which is very lethal.
Nerve injuries could be seen mainly at the following sites: brachial plexus, stellate ganglion, and phrenic nerve. Chronic pain syndromes are also possible.
Tissue Trauma: Trauma due to needle, guidewire, dilator, or even the catheter had been reported. Dilator-induced tissue or vascular injuries are much more important since the dilators usually cause more harmful injuries than other items used in CVC kits. However, large-bore lumen catheters could lead to tissue injuries; catastrophic results should be anticipated if central vessels or cardiac chambers are injured or, in worst conditions, “ruptured” (see above).
Catheter or guidewire embolization usually mandates emergent consultation with an interventionist or a surgeon. During CVC placement, guidewire could be introduced inside the venous system unintentionally, so it is necessary that the clinician take control of the distal end of the guidewire before introducing the whole catheter into the venous system. Also, there are reports of partial catheter fracture; both of these states could lead to catheter or guidewire embolization into the venous system, cardiac chambers, or even the pulmonary veins, being dislodged in between the lung tissues. The medical team should check any catheter fractures continually in patients admitted in intensive care wards, especially when the patients are awake and there is the possibility for spontaneous changes in body position. Another critical time for occurrence of this complication is at the time of CVC removal, which mandates careful examination of the whole CVC length, and check for its tip to be intact after withdrawing from the patient.
4.3.5.2 Thromboembolic Complications
These complications are more common in patients with femoral CVC and are lowest in subclavian approach. The primary nidus can change to an infectious complication or may dislodge to the pulmonary vasculature; usually these complications need surgical removal.
4.3.5.3 Infectious Complications
Infectious complications are among the most common late complication of CVC, with 30–50 % mortality. Strict adherence to aseptic techniques during catheter placement is cornerstone of all preventing strategies for CVC infection. Using subclavian approach, application of catheters coated with chlorhexidine and silver sulfadiazine or rifampin and minocycline, and selection of single-use catheters have been demonstrated as methods to decrease the rate of CVC-related infection. Also, the site of catheter insertion could affect the incidence of infectious complications being less in subclavian approach than other routes, though some controversies exist.
4.3.5.4 Other Complications
Arrhythmia is a very frequent and usually benign complication during CVC insertion especially in Seldinger technique (due to the guidewire); however, there are reported cases of malignant arrhythmias exactly at the time of CVC insertion, so careful attention to patient’s rhythm and hemodynamic status is a main concern during CVC insertion. However, the catheter itself could induce arrhythmias due to its physical effects if not in an appropriate location, which could happen intra- or postoperatively. Careful attention in the length of catheter entry is very important. Also, objective catheter tip confirmation by CXR is an essential job, confirming the CVC tip above the carina bifurcation; otherwise, the catheter should be a bit withdrawn in such a way that its tip is not far beyond carina.
Bleeding and air emboli are often related to CVCs with large-bore lumens, though other types of CVC may also have this side effect. Forgetting to secure the lumen ports could result in unnoticed bleeding. If the patient is hypovolemic, this complication would be manifested as air emboli entering the central venous circulation through the nonsecured lumen. Air embolism is also possible during two other situations besides unnoticed nonsecure ports; one is during catheter insertion, which is usually accompanied with small volumes of air going through the needle to the venous system, while the other situation is after CVC withdrawal which could be accompanied with air embolization through the skin lumen and subcutaneous tunnel created by after catheter removal. A compressed dressing with proper sealing could prevent both potential air embolism and CVC site bleeding after CVC removal.
Misinterpretation of data and errors in interpretation of data could lead to erroneous results and potential untoward clinical outcomes. For prevention of such erroneous judgments, the clinicians often do not rely only on the results of central venous pressure recordings; instead, the trend of CVP changes, curve of CVP, other measurements of filling pressures, jugular venous pressure figures, etc., are added to the online readings of CVP to prevent such unwanted events (Daily et al. 1970; Knopp and Dailey 1977; Berghella et al. 1979; Tyden 1982; Ferguson et al. 1988; Siradovic et al. 1988; Holmes et al. 1989; Kolodzik 1989; Meyer 1990; Heiss 1992; Mansfield et al. 1994; Tesio et al. 1994; English et al. 1995; Reed et al. 1995; Kuhn 2001; Merrer et al. 2001; Asheim et al. 2002; Keenan 2002; Woodrow 2002; Hind et al. 2003; Unal et al. 2003; Arai and Yamashita 2005; Botha et al. 2006; Di Iorio et al. 2006; Karakitsos et al. 2006; Ash 2007; Chen et al. 2007; Harrigan et al. 2007; Trieschmann et al. 2008; Brusasco et al. 2009; Kujur et al. 2009; Surov et al. 2009; Ishizuka et al. 2010; Kunizawa et al. 2010a, b; Omar et al. 2010; Furuya et al. 2011; Kang et al. 2011; Kim et al. 2011, 2012; McGee et al. 2011; Uchida et al. 2011; Urban et al. 2011; Boyce 2012; Calabria et al. 2012; Chopra et al. 2012; Ge et al. 2012; Godoy et al. 2012; Guleri et al. 2012; Hewlett and Rupp 2012; Lee and Kamphuisen 2012; Liang et al. 2012; Linnemann and Lindhoff-Last 2012; Marik et al. 2012; Miller and Maragakis 2012; Parienti et al. 2012; Pikwer et al. 2012; Reems and Aumann 2012; Stone et al. 2012; Turi et al. 2013; Vats 2012; Walser 2012; Zhou et al. 2012; Gibson and Bodenham 2013).
4.4 Pulmonary Artery Pressure Monitoring
4.4.1 History
Pulmonary artery catheter (PAC) was reported first in 1970 by Dr. Swan and Dr. Ganz; hence, the catheter is frequently known as the Swan-Ganz catheter. At that time, this catheter could introduce a new field of online cardiovascular monitoring, controlling the response to therapies and clinical data collection to the clinical world which was not accessible in such a novel way till that time. Due to its novel data, its use was rapidly increased during the following years both inside the operating rooms and intensive care units for cardiac and noncardiac patients. So PAC could help us retrieve a number of useful data which are not retrievable by other monitoring devices like CVC-recorded pressure monitoring; these data are used for diagnostic and/or therapeutic uses, i.e., assessment of the effects of therapies.
4.4.2 Clinical Outcome of PAC Usage
During the recent years, there are an increasing number of evidence which question the clinical and final clinical outcome of using PAC, creating an overwhelming load of controversies in using this monitoring device, though some studies have confirm the usefulness of PAC in decreasing mortality in critical patients. Some results of these studies are discussed here briefly.
The negative studies claim that PAC could not decrease the overall mortality or hospital length of stay in critical care patients; also, right heart catheterization in ICU causes increased risk for severe end-organ complications and increased mortality, costs, and length of stay, in such a way that some studies have recommended withdrawal of PAC from current clinical practice in adult ICU patients as a cost-effective strategy; on the other hand, currently, many of the clinicians are seeking newer, less invasive monitoring to be validated and used in practice instead of PAC; among them, transesophageal echocardiography (TEE) could be named as one of the most useful devices for such an application that gives us the needed online data without potential risks of PAC; finally, these data at least recommend us not to use PAC as a routine monitoring, especially in low-risk patients; on the other hand, the positive studies confirm the beneficial role of PAC in decreasing mortality.
4.4.3 Indications for PAC Use
PAC is clinically indicated for assessment of these main variables and their response to therapeutic interventions during a wide range of different disease entities:
Loading status, mainly including right heart failure, pulmonary dysfunction, pulmonary hypertension, severe left ventricular failure needing extra treatments like intra-aortic balloon pump, or shock (septic, cardiogenic, or non-cardiogenic)
Main hemodynamic parameters of the right heart and the lung, including pulmonary artery pressure (PAP), pulmonary artery occlusion pressure (PAOP), pulmonary vascular resistance (PVR), and mixed venous oxygen saturation (SvO2)
Monitoring and assessment of cardiac output (CO)
However, risks and benefits of PAC insertion should be assessed before its utilization. Often in low- to moderate-risk cardiac patients, the risks outweigh the benefits, and it is not recommended to use PAC as a “routine” monitoring in such cardiac surgery patients, especially if there is not enough experience and sufficient skill in insertion of PAC and interpretation of its data. In other words, like many other diagnostic devices and technologies, “appropriate patient, sufficient skill in PAC insertion and data analysis, and, finally, appropriate setting” should be present before utilization of PAC.
4.4.4 Contraindications of PAC
These are usually due to anatomical causes and include:
Tricuspid valve stenosis
Pulmonary valve stenosis
Previous mechanical prosthesis in any of the two above valves
Current endocarditis in any of the two above valves
Tumor and/or mass in right atrium or right ventricle
Thrombosis in right atrium or right ventricle
Tetralogy of Fallot due to the potential hyper-reaction of the pulmonary artery in such patients to mechanical stimuli causing spasm and cyanotic spells
Current underlying severe arrhythmia mandating full preparedness to treat any hemodynamic derangement
Newly inserted pacemakers which could lead to displacement of the pacemaker wire
Contraindications to insertion of CVC (see the previous section)
4.4.5 Technical Considerations for PAC Insertion
The basic principles for PAC insertion are in essence similar to CVC insertion; however, a few more points should be considered which are discussed here:
The most preferred approach is right internal jugular vein, while subclavian and femoral veins are not routinely used especially for cardiac surgery patients.
Guiding the catheter through its path in order to reach to pulmonary artery is used by three ways: the curve of the wave on monitor and its trend, fluoroscopic assessment of catheter tip, and electrocardiography (ECG)-guided approach; however, in intensive care unit, usually the curve of the wave is used for catheter insertion and controlled by chest X-ray.
Changing the patient position to a head-down and right lateral tilt helps the tip of the PAC to progress from right atrium (RA) to right ventricle (RV) and pulmonary artery (PA).
An introducer sheath is inserted first, which is mainly like a CVC; this sheath is the conduit for passage of PAC and lets us move PAC back and forth; however, it is a relatively large-bore CVC, nearly 8.5–9 F, and so mandates vigorous caution during its insertion.
The catheter is 110 cm length and usually 7–8 F. having length markers at 10 cm intervals for accurate and careful back-and-forth movements of the catheter.
There is a sterile plastic cover on the catheter; this plastic cover should be attached firmly to the distal head of the introducer sheath to prevent contamination, though it is not fully protective and mandates strict adherence to infection prevention methods and, more importantly, avoidance of unnecessary back-and-forth movements.
Before passing the catheter tip through the introducer sheath and advancing it to the right heart, zeroing of the catheter with the monitoring system at the level of the right atrium should be done while the patient is at supine position; also, the tip of PAC should be raised about 30 cm, and the pressure should be checked with the monitor which equals 22 mm Hg.
PAC has usually 4–5 lumens for the following purposes (demonstrated in Fig. 4.4):
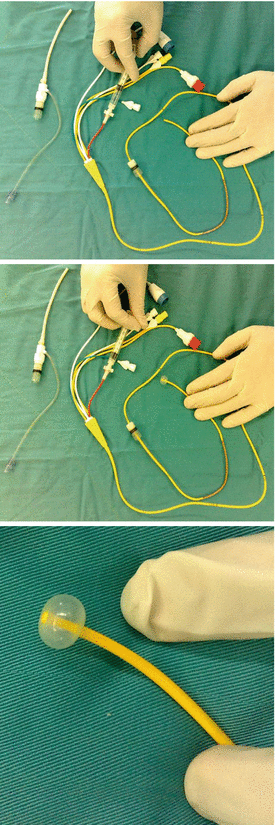
Fig. 4.4
PAC ingredients: note the tip of the balloon in three subsequent figures to be inflated; the 3rd figure demonstrates a filled balloon in large view
1.
Distal lumen near the tip of catheter for measurement of pulmonary artery pressure.
2.
A balloon located 4–5 cm proximal to the tip of catheter and 1.5 mL volume which should be filled for “flow-directed movement of the catheter”; its related lumen should be attached to a specific 2 mL syringe, and the balloon should be checked for symmetrical filling before insertion of PAC; also, the balloon should not be filled when PAC tip is in wedge position to prevent unwanted injuries in lung tissue and pulmonary arterial branches.
3.
The most proximal lumen opening nearly 30 cm proximal to the tip of catheter used for measurement of central venous pressure or RA pressure.
4.
The 4th lumen having a thermistor for measurement of cardiac output using thermodilution method.
5.
Often the 5th lumen being used for assessment of mixed venous blood oxygen saturation (SvO2).
When advancing PAC through introducer sheath from the right internal jugular approach, we should have different related curve patterns at related distance intervals, i.e., for CVP (10–15 cm), RA pressure (15–25 cm), RV pressure (30–35 cm), PA pressure (40–45 cm), and wedge pressure (45–55 cm) as described below and demonstrated in Fig. 4.5:
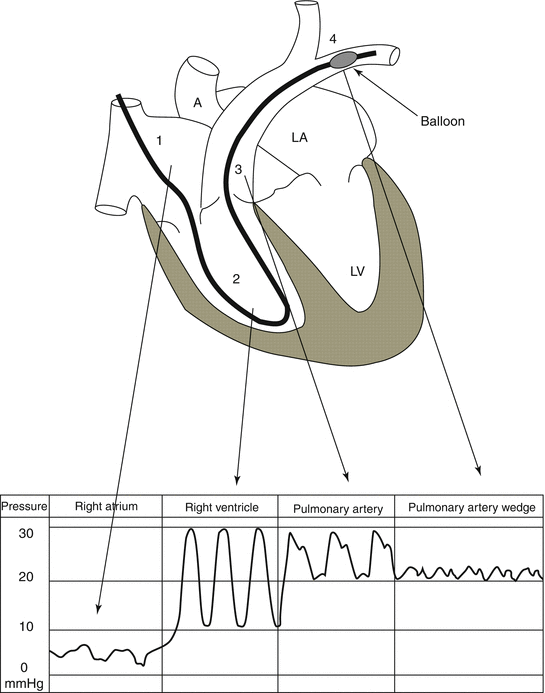

Fig. 4.5
A schematic presentation of the PAC course and its related pressure waveforms in cardiac chambers, pulmonary artery, and main left pulmonary artery
1.
Central venous pressure curve, demonstrating the tip of the catheter being located in central veins and/or RA, for CVP at 10–15 cm and for RA pressure at about 15–25 cm.
2.
The catheter tip passes through the tricuspid valve to go to the right ventricle (RV); a significant increase in systolic deflection is seen, and the diastolic deflection does not change so much; this part of the curve belongs to RV when PAC tip goes in as much as 30–35 cm.
3.
With catheter progress through the pulmonary valve to reach the entry of pulmonary artery (PA), a dicrotic notch is seen in the point just after the peak of systole, while the bottom of the pressure wave curve increases suddenly showing the diastolic pressure of PA which is higher than the diastole of RV and resembles the wave contour of the systemic arterial pressure; of course in a smaller scale; so PA pressure appears when PAC tip goes in about 40–45 cm.
4.
Catheter progression as much as about 5 cm causes another new pattern in wave contour which would be nearly at 45–55 cm from PAC tip; here, there would be another change in the wave contour which indicates pulmonary artery wedge pressure (PAWP) or pulmonary artery occluding pressure (PAOP) which could usually be demonstrated on the monitor screen play after PAC balloon dilatation leading to wedge location of the tip of catheter; of course, PAC tip should not stay in wedge position for more than a few minutes to prevent unwanted complications like rupture of the pulmonary arterial branches or segmental necrosis of lung tissue; frequent withdrawal of PAC as much as 3–5 cm prevents PAC tip lodging, hence preventing such problems.
5.
If these curves are not seen in the above length intervals, there is a real chance of catheter coiling, a dangerous complication of PAC discussed in the next paragraph; for prevention of such an event, strictly following the curves is a useful preventive strategy.
4.4.6 Complications of PAC
In 2003, the American Society of Anesthesiologists’ task force on PAC (2003) stated that “overall deaths attributable to PAC are 0.02–1.5%” and also classified PAC complications in three main categories:
1.
PAC complications due to complications of CVC, i.e., “arterial puncture, postoperative neuropathy (pain and sensation deficit), air embolism (air in blood vessels), and pneumothorax (air outside the lungs), reported in less than 3.6 %.”
2.
The complications mainly related to the mechanical effects of the catheter itself, i.e., “severe dysrhythmias, right bundle branch block, and complete heart block, seen in 0.3–3.8 %.”
3.
The complications related to persisting catheter in place, i.e., “rupture of the pulmonary artery and its attributed death, infarction of the pulmonary tissue, catheter-related sepsis, positive catheter tip culture, valvular/endocardial vegetation, thrombophlebitis, mural thrombosis, and thrombosis of the veins, i.e., clots in vein, occurring between 0.03 and 3 %.”
Sufficient skill, enough experience, and having a high index of suspicion and vigilance in detection of any complication may help the clinician prevent these potentially deadly complications or diagnose them as early as possible. The following comments should be remembered regarding the complications of PAC:
Atrial or ventricular arrhythmia, often in the form of ventricular premature contractions, is seen during catheter passage from RV; these arrhythmias are usually benign; head-up and right lateral position might decrease the incidence of this complication.
PAC could lead to right bundle branch block (RBBB); so in patients with underlying left bundle branch block (LBBB), complete atrioventricular block (AV block) would be possible; though not frequent, it could be lethal.
Pulmonary artery injuries might cause lethal pulmonary artery perforation or rupture mandating emergent surgery or stenting of the rupture; late pulmonary artery false aneurysm is also reported.
Pulmonary infarction due to catheter dislodgement in segmental or bronchial pulmonary arterial branches is another potential complication.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


