Cardiovascular Medicine—Essential Pharmaceuticals
Cardiovascular disease (CVD) accounts for nearly 50% of all death in Western societies and 25% of all death worldwide. Furthermore, cardiac medications rank second in frequency of use worldwide, after antibiotics. Medical therapy plays an essential role, not only in the management of CVD but also in prevention.
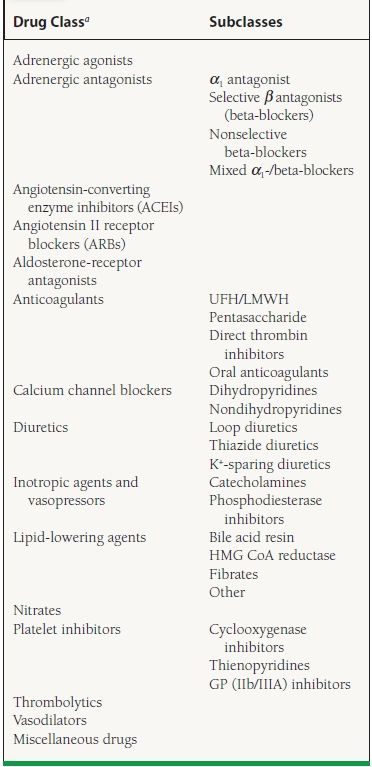
Pharmacology accounts for about 12% of the questions on the Cardiology Boards. The topic is vast and may be approached in various ways. Pharmacokinetics and dynamics, drug interactions, and antiarrhythmics are addressed in other chapters. In this chapter, we cover most of the available cardiovascular drugs used in clinical practice in the United States. We review names (brand and generic), starting and maximum doses, mechanisms of action, labeled and unlabeled indications, and side effects. When appropriate, we comment on the major clinical trials involving the particular drug. The classes and drugs are classified alphabetically according to mechanistic class and/or therapeutic class, as shown in Table 61.1.
TABLE
61.1 Major Classes of Cardiac Medications, Listed Alphabetically
aAntiarrhythmics are covered in Chapter 10.
ADRENERGIC AGONISTS
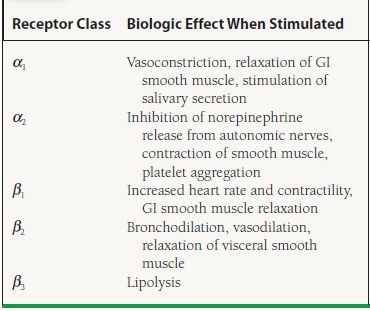
Before detailing specific medications, we review basic information on adrenergic receptors that is useful for understanding the mechanism and side effects of this class as well as classes discussed subsequently. The adrenoceptors are classified into alpha (α) and beta (β) subtypes. There are two main subtypes of α-adrenoceptors, α1 and α2, and three main types of β-adrenoceptors, β1, β2, and β3. The primary effects of receptor activation are shown in Table 61.2. The adrenergic agonists of cardiac interest in this section are the α2 agonists, which act centrally. In later sections, we discuss other adrenergic agonists that stimulate α and β receptors, such as epinephrine, norepinephrine, dobutamine, and other inotropes and pressors.
TABLE
61.2 Main Effects of Receptor Activation of Adrenoceptors
The α2 Agonists (Clonidine, Guanabenz, Guanfacine)
Mechanism of Action
The α2 receptors are presynaptic and are found in the central nervous system (CNS). Stimulation of the a2 receptors decreases sympathetic outflow from the CNS, thus lowering blood pressure and, in some patients, heart rate.
Side Effects
Common side effects include sedation, dry mouth, hypotension, dizziness, sexual dysfunction, bradycardia, nausea, headache, and depression. Abrupt withdrawal of therapy causes rebound hypertension. Rebound hypertension can be severe with concurrent administration of beta-blockers secondary to unopposed α1-receptor stimulation.
Indication and Precautions
Clonidine (Catapres and Catapres-TTS) Clonidine is labeled for use in hypertension. For patients with severe renal insufficiency (i.e., CrCl <10 mL/min), doses should be reduced by 25% to 50%. Transdermal patches are replaced weekly.
Methyldopa (Aldomet) (Oral); Methyldopate HCL (Intravenous) Methyldopa is labeled for use in hypertension and hypertensive emergency (IV only) and is one of the few drugs that can be used for hypertension in pregnant women. In addition to the general side effects of α2-receptor agonists, methyldopa has specific side effects: peripheral edema, hemolytic anemia, drug fever, systemic lupus erythematosus (SLE)-like syndrome, nightmares, hepatocellular injury, hepatitis (rare), and anxiety. Positive Coombs tests occur within 6 to 12 months in 10% to 20% of patients.
ADRENERGIC ANTAGONISTS
This section is divided according to the various subgroups of adrenergic antagonists, based on the adrenergic receptors they block, and include α1-receptor antagonists, β-receptor antagonists, and nonselective α/β antagonists.
α1-Receptor Antagonists
Selective α1, Antagonists: Doxazosin (Cardura), Prazosin (Minipress), Terazosin (Hytrin)
Mechanism of Action Selective α1-receptor antagonists act peripherally and lead to arterial and venous vasodilation.
Side Effects Side effects of α1-receptor antagonists include orthostatic hypotension, dizziness, light-headedness, drowsiness, headache, dry mouth, and malaise. The first dose should be given at bedtime to limit effects associated with orthostatic hypotension. Tachyphylaxis may develop with long-term administration in patients with heart failure (e.g., prazosin).
Indications and Precautions These drugs are labeled for use in hypertension and benign prostatic hypertrophy (BPH) (terazosin and doxazosin).
Major Clinical Trials
VHeFT I.1 In this trial, hydralazine combined with nitrates and prazosin was compared to placebo for the treatment of heart failure. No effect on the primary endpoint of mortality was observed, and no difference in left ventricular (LV) ejection fraction was observed when prazosin was compared to placebo.
ALLHAT.2 This trial was designed to evaluate different antihypertensive medications to reduce cardiovascular events. An interim analysis 2 years before the final publication demonstrated that patients receiving doxazosin had a significantly higher rate of stroke cardiovascular events, and the rate of congestive heart failure (CHF) was two times higher. The doxazosin arm was stopped early as a result of the findings.
Nonselective α Antagonists: Phentolamine (Regitine)
Mechanism of Action Nonselective α-adrenergic antagonist with similar affinities for α1 and α2 receptors, producing vasodilation and an increase in heart rate
Side Effects Side effects of these drugs include hypotension, tachycardia, arrhythmias, angina, and nausea/vomiting/diarrhea. They may exacerbate peptic ulcer disease (PUD) and produce nasal congestion.
Indications and Precautions Phentolamine is labeled for use in hypertensive crisis in patients with pheochromocytoma and for treatment of skin necrosis in patients with norepinephrine, dopamine, epinephrine, and phenylephrine extravasation.
β-Receptor Antagonists
Beta-blockers were first discovered in 1958 (dichloroiso-prenaline). The effects produced depend on the degree of endogenous sympathetic activity and are less dramatic at rest. Beta-blockers are classified as selective and nonselective as well as having α1-blocking properties.
Mechanism of Action Nonselective beta-blockers antagonize both β1 and β2 receptors, inhibiting the effects of catecholamines on these receptors. Cardiovascular effects include decreases in contractility and heart rate. Noncardiovascular effects mediated through β2 blockade include increased peripheral vascular resistance or bronchospasm. Selective beta-blockers antagonize β1 receptors to a greater extent than β2 receptors, when administered in typical or usual doses. Cardiovascular effects are the same as with nonselective beta-blockers. Both classes generally lead to a decrease in blood pressure, sinus node automaticity, conduction through the atrioventricular (AV) node, and increased AV nodal refractoriness. The antiarrhythmic properties are a class effect. Other pharmacologic properties of selective beta-blockers include intrinsic sympathomimetic activity (ISA). Agents that have ISA are partial β agonists during low catecholamine states, preventing resting bradycardia; however, they act as full agonists when endogenous catecholamine levels increase. Beta-blockers without ISA activity have been shown to decrease recurrent myocardial infarction (MI), sudden death, and overall mortality in acute-MI survivors. They also have been shown to reduce mortality and hospitalizations secondary to heart failure. A new beta-blocker, nebivolol, is a selective β1 antagonist that also reduces systemic vascular resistance (SVR) by producing an endothelium-derived nitric oxide (NO)-dependent vasodilation. A list of the various beta-blockers that are used is provided in Table 61.3.
TABLE
61.3 Pharmacokinetics of Commonly Used Beta-Blockers
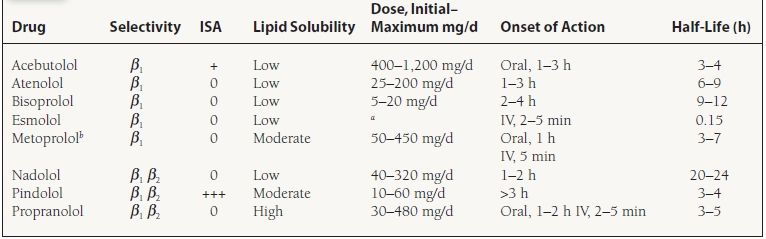
aEsmolol is an IV-formulated drug. The loading dose is 500 µg/kg given over 1 min. Then, the maintenance dose is 25–300 µg/kg/min.
bMetoprolol is available as an immediate-release preparation and as an extended-release preparation. The extended-release product is labeled for both hypertension and heart failure.
ISA, intrinsic sympathomimetic activity.
Side Effects Side effects of these drugs include fatigue, bradycardia, heart block, bronchospasm, depression, lipid abnormalities, masking the symptoms of hypoglycemia, a rebound effect with abrupt discontinuation, precipitation of heart failure, and impotence.
Indications and Precautions Indications for beta-blockers include hypertension, ischemic heart disease, acute myocardial infarction (AMI), stable and unstable angina (UA), heart failure, arrhythmia, and stable heart failure (see Table 61.3). Absolute contraindications include hypersensitivity to beta-blockers, asthma, heart block greater than first degree, insulin-dependent diabetics with frequent hypoglycemic episodes, and overt heart failure. Relative contraindications include chronic obstructive lung disease, diabetes mellitus, and severe peripheral arterial disease. Beta-blockers have significant interactions with other drugs, including medications that slow AV nodal conduction such as digoxin, diltiazem, and verapamil, as well as nonsteroidal anti-inflammatory drugs (NSAIDs), other antihypertensive medications, other negative inotropic agents, rifampin, phenobarbital, phenytoin, cholestyramine, and colestipol, to name a few.
Combined α-/β-Receptor Antagonists
Mechanism of Action The combination preparations are specific α1-receptor antagonists, but nonselective b-receptor antagonists. Labetalol is 7:1 selective b to a receptor in the IV preparation and 3:1 in the oral preparation.
Side Effects Refer to side effects of beta-blockers.
Indications and Precautions The labeled indication of labetalol is hypertension. Carvedilol has labeled indications for hypertension, as well as LV dysfunction following MI (clinically stable patients), and mild to severe chronic heart failure. Refer to precautions for beta-blockers.
Major Clinical Trials
MERIT-HF.3 Metoprolol CR/XL improved survival and New York Heart Association (NYHA) functional class and reduced the number of hospitalizations and days in the hospital due to worsening heart failure.
CIBIS-II.4 This trial demonstrated that bisoprolol therapy was well tolerated and reduced mortality and hospitalization rates in patients with stable CHF.
CAPRICORN.5 This trial showed that long-term therapy with carvedilol, in addition to angiotensin-converting enzyme inhibitor (ACE-I) and standard therapy, reduced mortality and recurrent MI in stable patients with LV systolic dysfunction after acute MI.
COPERNICUS.6 This trial studied the effect of carvedilol on survival in patients with severe chronic heart failure. Carvedilol reduced mortality in patients with severe CHF by 34% and also reduced the number of days spent in hospital because of CHF and for any cause.
COMET.7 Carvedilol improved survival and LV ejection fraction to a greater degree than immediate-release metoprolol during long-term therapy for heart failure.
BHAT.8,9 The Beta-blocker Heart Attack Trial, using propanolol, and the Norwegian Multicenter Study Group, using timolol, both showed a reduction in mortality rate, reinfarction rate, or both with the use of the beta-blocker.
ANGIOTENSIN-CONVERTING ENZYME INHIBITORS
The renin–angiotensin system (RAS) is a hormonal system that interacts very closely with the sympathetic nervous system, angiotensin II formation, and aldosterone secretion. It has an essential role in sodium and volume management. Renin, an enzyme, is secreted by the juxtaglomerular apparatus located in the wall of the arteriole of the glomerulus. It is secreted secondary to multiple stimuli, low sodium concentration of the fluid in the distal tubule, stimulation of the β-adrenoceptors, and circulating prostacyclin and NO, as shown in Figure 61.1.
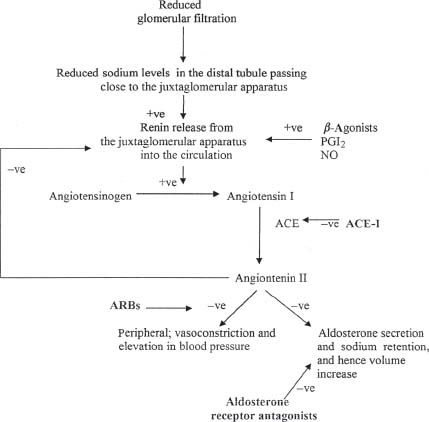
FIGURE 61.1 The renin–angiotensin–aldosterone pathway and mechanism of action of ACE-I and ARBs. ACE is a membranebound enzyme on the surface of endothelial cells abundant in the lung (+ve, positive or stimulatory effect; –ve, negative or inhibitory effect).
The RAS pathway is important in the pathogenesis of heart failure and hypertension; hence all aspects of this pathway are targets for therapy in CHF and hypertension, whether in renin release such as beta-blockers, angiotensin II formation such as ACE-I, or angiotensin II blockade such as angiotensin II receptor blockers (ARBs) and aldosterone antagonists.
Mechanism of Action
ACE-I block the conversion of angiotensin I to angiotensin II by inhibiting ACE, as shown in Figure 61.1. In response to this blockade, there is decreased formation of angiotensin II, leading to vasodilatation. Also, there is a decrease in the breakdown of bradykinins, which may produce additional vasodilatory effects.
Side Effects
Side effects include cough (because of decrease in breakdown of bradykinin), acute renal failure (particularly if there is bilateral renal artery stenosis), angioedema, hyperkalemia, proteinuria, hypotension (which needs to be monitored after the first dose and in water-depleted patients), headache, rash, neutropenia/agranulocytosis, and dizziness.
Angioedema is rare but may occur at any time; when it does occur, however, it is usually early in therapy. Severe hypotension may be seen in volume-depleted patients; hence the volume status of a heart failure patient should be carefully assessed before initiating therapy. Neutropenia or agranulocytosis has been reported with many ACE-I and is usually associated with collagen vascular disorders, higher doses, and renal dysfunction.
Indications and Precautions
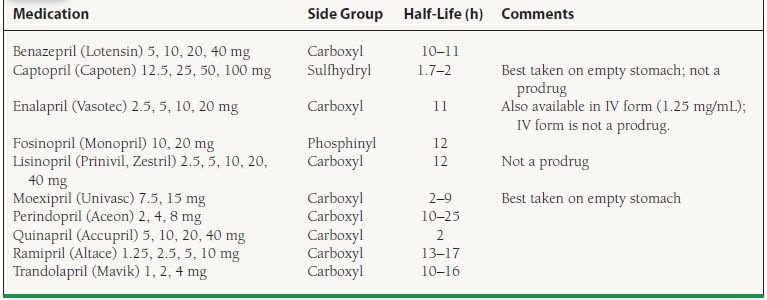
ACE-I are labeled for use in hypertension, heart failure, LV dysfunction, post-AMI, and diabetic nephropathy (Table 61.4). ACE-I are contraindicated in pregnancy and in patients with bilateral renal artery stenosis or unilateral renal artery stenosis in patients with a solitary kidney.
TABLE
61.4 Preparations and Dosing of Available ACE-I
Major Clinical Trials
SAVE.10,11 The SAVE trial showed that the long-term use of captopril in patients with asymptomatic LV dysfunction following acute MI was associated with lower mortality and morbidity.
GISSI-3.12 This trial showed that lisinopril therapy reduced mortality and improved outcome after MI. The mortality benefit manifested primarily during the early phase (6 weeks), whereas the LV remodeling benefit manifested later.
TRACE.13 The TRACE trial update confirmed that trandolapril reduced death and major cardiovascular complications in diabetic patients after infarction.
HEART.14 The HEART trial showed that in patients with acute anterior MI, early use of ramipril (titrated to 10 mg) attenuated LV remodeling and resulted in swift LV recovery.
AIREX.15 This trial showed that beneficial effects of ramipril started early after MI in patients with heart failure, and that the benefit was sustained over several years.
CONSENSUS.16 CONSENSUS studied the effects of enalapril on mortality in severe CHF. Enalapril reduced mortality and improved symptoms in severe CHF, when added to conventional therapy.
V-HeFT II.17 This trial compared enalapril with hydralazine–isosorbide (H-ISDN) for the treatment of CHF. Mortality with enalapril was significantly lower than in the H-ISDN group.
Stop-Hypertension-2.18 This trial showed that ACE-I and calcium channel blockers have similar efficacy in prevention of cardiovascular mortality compared to older-generation antihypertensive drugs (diuretics and beta-blockers) in elderly patients. ACE-I was associated with less MI and CHF than calcium channel blockers, but not compared to conventional therapy with diuretics and beta-blockers.
ANGIOTENSIN-II RECEPTOR BLOCKERS
Mechanism of Action
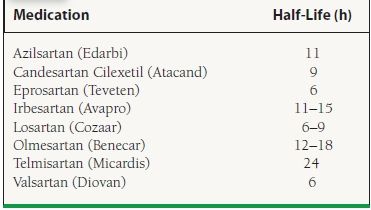
ARBs block angiotensin II (A-II) from binding to angiotensin II type 1 receptors (AT1), thereby inhibiting the vasoconstrictor and aldosterone-secreting effects of angiotensin II (see Fig. 61.1; Table 61.5). ARBs do not alter the metabolism of bradykinin or neuropeptides, so they should not cause side effects related to this effect, such as cough. However, there are case reports of angioedema occurring with ARB therapy.
TABLE
61.5 Formulations of ARBs
Side Effects
Side effects include hypotension, acute renal failure (particularly if there is bilateral renal artery stenosis), hyperkalemia, proteinuria, hypotension, dizziness, headache, and angioedema (rare).
Indications and Precautions
The labeled use for all ARBs is hypertension; however, both candesartan and valsartan are also labeled for heart failure. Candesartan is labeled for cardiovascular mortality reduction for patients with reduced LV dysfunction post-MI. Losartan is also labeled for hypertension in patients with LV hypertrophy, as well as prophylaxis of diabetic nephropathy in patients with a history of hypertension. Additionally, irbesartan also carries an indication for prophylaxis against diabetic nephropathy in patients with a history of hypertension.
Major Clinical Trials
ELITE-II.19 This trial demonstrated that losartan was not superior to captopril in reducing mortality in patients with CHF who were >60 years of age.
CHARM.20 This trial had multiple arms. The CHARM-alternative looked at candesartan in patients with symptomatic heart failure who were intolerant of ACE-I. Patients were randomized to either candesartan or placebo. There was decreased risk of cardiovascular death or hospitalization from heart failure in the candesartan group compared to the placebo group. The CHARM-added trial randomized patients with symptomatic heart failure who were already on ACE-I to either candesartan or placebo. In contrast to the results of Val-HeFT, mortality was significantly reduced by addition of an ARB to an ACE-I compared to the ACE-I alone. The CHARM-preserved arm of the study looked at patients with symptomatic heart failure with preserved ejection fraction. They were randomized to either candesartan or placebo. There was no difference in the primary endpoint (cardiovascular death or hospitalization secondary to CHF).
LIFE.21 This trial showed that losartan was more effective than atenolol in preventing death, cardiovascular accidents (CVA), and MI as a combined endpoint. In terms of single outcomes, the reduction in CVA was significant, whereas the reduction in death or MI was only a trend without statistical significance.
Val-HeFT.22 This trial showed that valsartan reduced the combined endpoint of death and morbidity and improved symptoms and signs of CHF. The benefit was seen only in patients who were not receiving ACE-I. In patients who were on ACE-I and beta-blockers, the addition of valsartan was associated with increased mortality.
RESOLVD.23 This trial showed that both candesartan and enalapril were effective, safe, and tolerated in the treatment of CHF. Combination of candesartan and enalapril reduced LV dilatation greater than either agent alone.
RENAAL.24 This trial showed that losartan preserved renal function in patients with type II diabetes and diabetic nephropathy, as well as decreasing hospitalization for CHF.
ALDOSTERONE RECEPTOR ANTAGONISTS
Mechanism of Action
As shown in Figure 61.1, this class of drugs antagonizes aldosterone at the mineralocorticoid receptor, consequently inhibiting aldosterone effects in the late distal convoluted tubule and cortical collecting duct, reducing sodium reuptake (hence the diuretic effect) and reducing potassium excretion (hence the side effect of hyperkalemia).
Side Effects
Side effects of spironolactone include hyperkalemia, hypotension, fatigue, rash, gynecomastia, amenorrhea, breast tenderness, sexual dysfunction, headache, nausea/vomiting, and diarrhea. With eplerenone, hyperkalemia is the predominant effect, with gynecomastia much less common. Drug–drug interactions are common with eplerenone, especially when concomitant medications increase serum potassium levels and when it is given with potent inhibitors of the CYP 3A4 isoenzyme system.
Indications and Precautions
Spironolactone is labeled for primary aldosteronism, edema, hypertension, severe heart failure (NYHA Class III-IV) to increase survival and reduce hospitalization when added to standard therapy, and hypokalemia associated with loop diuretics. Use at a dose of 25 mg daily as an adjunct therapy for patients with Class III to IV CHF. Eplerenone is labeled for use in hypertension and for treatment in patients with LV dysfunction after MI. It is important to point out that this class of drugs should be avoided in patients with renal dysfunction (creatinine >2.5 mg/dL) or hyperkalemia.
Major Clinical Trials
RALES.25 RALES found that the addition of spironolactone to standard-therapy ACE-I, loop diuretic, and digoxin reduced mortality and hospitalizations due to heart failure, with a 30% decrease in mortality in patients with NYHA Class III or IV heart failure.
EPHESUS.26 This trial was designed to assess the safety and efficacy of eplerenone in patients with CHF after acute MI. The study showed a 15% decrease in mortality in patients with CHF post-MI.
EMPHASIS-HF.27 This trial added eplerenone (up to 50 mg daily) or placebo to recommended therapy in patients with NYHA functional Class II heart failure and an ejection fraction of no more than 35%. Primary outcome was the composite of death from cardiovascular causes or first hospitalization for heart failure. For patients in the eplerenone group, primary outcome occurred in 249 patients (18.3%) and in 356 patients (25.9%) in the placebo group with a hazard ratio for primary outcome for eplerenone compared to placebo of 0.63 (95% confidence interval, 0.54 to 0.74; p < 0.001). Of note, serum potassium levels above 5.5 mmol/L occurred in 158 patients (11.8%) in eplerenone group compared to 96 patients (7.2%) in the placebo group, (p <0.001).
RENIN INHIBITORS
Mechanism of Action
Aliskiren is a direct renin inhibitor blocking the conversion of angiotensinogen to angiotensin I, which, in turn, decreases formation of angiotensin II.
Side Effects
Side effects include increased BUN and serum creatinine, hyperkalemia, hypotension, and angioedema.
Indications and Precautions
The labeled indication for aliskiren is for hypertension. This medication has not been studied in patients with severe renal impairment. It is recommend to avoid use in patients with worsening renal function, or renal artery stenosis (bilateral or unilateral).
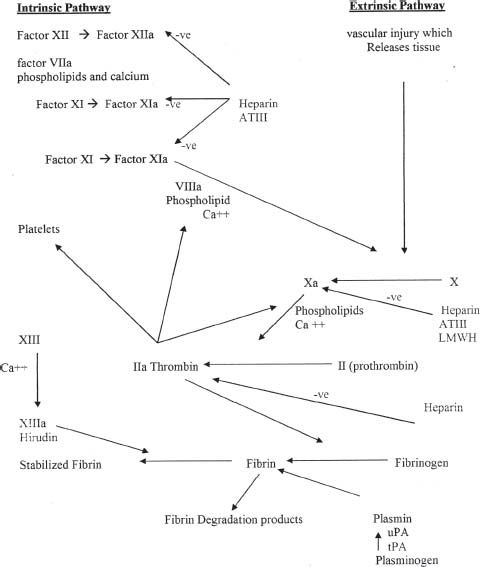
ANTICOAGULANTS
Anticoagulant drugs are subclassified into unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), direct thrombin inhibitors, and oral anticoagulants. A diagram of the coagulation cascade is shown in Figure 61.2.
FIGURE 61.2 The blood coagulation pathways, intrinsic and extrinsic, and the sites of impact of anticoagulant. When the arrow is not marked, it indicates either a positive effect of a factor on a step in the pathway or a transformation into another factor. The negative effect is marked on the relevant arrows (+ve, positive or stimulatory effect; –ve, negative or inhibitory effect).
Unfractionated Heparin
Mechanism of Action
UFH is a thrombin inhibitor that binds to antithrombin, increasing antithrombin’s activity to inactivate thrombin in addition to activated factors IX, X, XI, and XII.
Side Effects
Common side effects include bleeding, thrombocytopenia (benign type I and more severe type II, which may cause thrombosis), elevation in aspartate aminotransferase (AST)/alanine aminotransferase (ALT), osteoporosis (long-term therapy), and hyperkalemia.
Indications and Precautions
Heparin is administered subcutaneously or as an IV preparation. Generally, a weight-based nomogram is utilized that is titrated to a specific activated partial thromboplastin (aPTT) level. It is labeled for use in the treatment and prophylaxis of venous and arterial thrombosis. Heparin-induced thrombocytopenia (HIT) occurs in two forms, type I (non–antibody-mediated reaction) and type II (antibody-mediated reaction—typically IgG). Thromboembolic complications (e.g., deep vein thrombosis [DVT], MI, stroke) are associated with type II HIT and can be life-threatening. The incidence of thrombocytopenia is 10% to 15%, but the incidence of developing type II HIT is around 1% to 3%.
FACTOR XA INHIBITORS
Fondaparinux (Arixtra)
Mechanism of Action
Fondaparinux inhibits activated factor Xa through the neutralization capacity of antithrombin III.
Side Effects
Common side effects include bleeding, injection site–related bleeding, rash, and pruritus. Asymptomatic increases in AST and ALT may occur.
Indications and Precautions
Fondaparinux is used for prophylaxis of DVT in patients undergoing orthopedic or abdominal surgery. It is also indicated for treatment of acute pulmonary embolism (PE) and acute DVT (with or without PE). Fondaparinux is contraindicated in patients with a creatinine clearance <30 mL/min and in patients weighing <50 kg. As with direct thrombin inhibitors, there is no known antidote for fondaparinux.
Rivaroxaban (Xarelto)
Mechanism of Action
This prevents clot formation by selective inhibition of factor Xa.
Side Effects
Common side effects include bleeding (6%), ALT or AST elevation greater than three times upper limit of normal (3%).
Indications and Precautions
Rivaroxaban is indicated for thromboprophylaxis post knee or hip replacement. Avoid use in patients with moderate to severe hepatic dysfunction or severe (CrCl<30 mg/dL) renal dysfunction.
Major Clinical Trials
OASIS-5.28 This trial compared efficacy and safety of fondaparinux to enoxaparin in patients with UA or non–ST-elevation myocardial infarction (NSTEMI).
The primary efficacy endpoint composite of death, MI, and refractory ischemia at 9 days validated noninferiority of fondaparinux (5.8%) compared with 5.7% for enoxaparin (HR 1.01; 95% CI 0.90 to 1.13; p = 0.007). The safety endpoint of major bleeding at 9 days was reduced with fondaparinux (2.2%) compared to 4.1% with enoxaparin (HR 0.52; 95% CI 0.44 to 0.61; p < 0.001). Of note, major bleeding in patients with CrCl < 30 mL/min was less with fondaparinux (2.4%) compared to 9.9% with enoxaparin (p = 0.001). Patients in whom percutaneous coronary intervention (PCI) was performed who received enoxaparin 6 or more hours before the procedure were administered UFH during the procedure, which may lead to a higher risk of bleeding. Of concern was the rate of guiding-catheter thrombus formations that occurred in the fondaparinux group (29 episodes [0.9%] vs. 8 episodes [0.3%]).
OASIS-6.29 This study evaluated efficacy of fondaparinux with UFH in patients with acute ST-elevation myocardial infarction (STEMI). This was a randomized, double blind trial. There were two treatment groups, stratum 1, in which patients had no indication to receive UFH, and stratum 2, in which patients had an indication for UFH such as use of fibrinolytic therapy, patients not eligible for fibrinolysis but eligible for UFH, or primary PCI patients. Stratum 1 patients received fondaparinux for up to 8 days. Stratum 2 received either fondaparinux or placebo, with a control group receiving UFH for 24 to 48 hours. The primary endpoint at 30 days of death or reinfarction for fondaparinux was 9.7% compared to UFH or placebo group, which was 11.2% leading to a 14% risk reduction (0.86 HR; 95% CI, 0.77 to 0.96, p = 0.008). No difference was found between fondaparinux (2.1%) and the UFH or placebo group (1.8%) (0.83 HR; 95% CI, 0.64 to 1.06, p = 0.14) for major bleeding. For fondaparinux compared to control, significant benefit was found for patients without reperfusion (15.1% control, 12.2% fondaparinux, HR, 0.80; 95% CI, 0.65 to 0.98; p = 0.003), with fibrinolytic (13.6% vs. 10.9%; HR, 0.79; 95% CI, 0.68 to 0.92; p = 0.003), but not for the primary PCI patients (4.9% vs. 6.0%; HR, 1.24; 95% CI, 0.95 to 1.63; p = 0.12). With regard to PCI, as seen in OASIS-5, there was a significant rate of guiding-catheter thrombosis with fondaparinux (22 vs. 0; p < 0.001).
ROCKET AF.30 Rivaroxaban was compared to warfarin in patients with nonvalvular atrial fibrillation at moderate to high risk for stroke. Elevated risk was signified by history of stroke, transient ischemic attack, or systemic embolism, or at least two of the following: heart failure or ejection fraction of 35% or less, hypertension, age 75 or more, or diabetes mellitus. The composite of stroke (ischemic or hemorrhagic) and systemic embolism was the primary endpoint. This occurred in the rivaroxaban group (1.7% per year) versus in the warfarin group (2.3% per year) (HR for rivaroxaban group, 0.79; 95% CI, 0.66 to 0.96; p <0.001 for non-inferiority). Major bleeding was similar between the groups (3.6% and 3.4%, p = 0.58). Rates of intracranial hemorrhage were lower in the rivaroxaban group (0.5% vs. 0.7% per year; HR, 0.67; 95% CI, 0.47 to 0.93). Major bleeding from a gastrointestinal site was significantly higher for rivaroxaban (3.2% vs. 2.2, p < 0.001). Of note, patients in the warfarin group were in therapeutic range for INR 55% of the time, which is lower than previous studies (range, 64% to 68%).
Direct Thrombin Inhibitors: Bivalirudin (Angiomax), Lepirudin (Refludan), Argatroban (Argatroban), Dabigatran (Pradaxa)
Mechanism of Action
These drugs bind directly to thrombin (factor IIa), causing it to be inactivated (see Fig. 61.2). Bivalirudin, dabigatran, and argatroban bind directly and reversibly, whereas lepirudin binds directly and irreversibly.
Side Effects
Common side effects include bleeding. Patients on lepirudin may develop antibodies against lepirudin that decrease the elimination of lepirudin, hence increasing the duration of activity. Recent data demonstrated that patients may develop anaphylactic reactions with administration of lepirudin, and the occurrence is higher in patients who have previously been treated with the drug. Dabigatran has an increased incidence of dyspepsia (11%), but absorption of dabigatran is decreased by 20% to 25% when given in combination with proton pump inhibitors.
Indications and Precautions
Bivalirudin, lepirudin, and argatroban are given intravenously, and dabigatran is available orally. They are listed in Table 61.6. The labeled indication for bivalirudin is for percutaneous transluminal coronary angioplasty (PTCA) in the setting of UA. Dabigatran is indicated for prevention of stroke and systemic embolization in nonvalvular atrial fibrillation. There are no reversal agents for the direct thrombin inhibitors.
TABLE
61.6 Preparations, Dosing, and Indications for Direct Thrombin Inhibitors
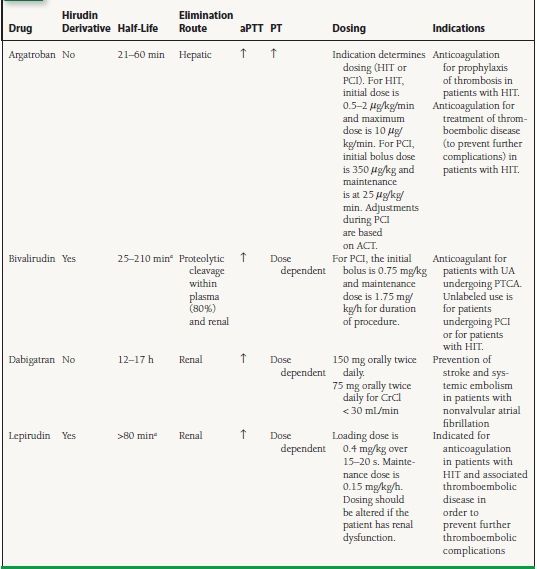
aHalf-life depends on renal function.
aPTT, activated partial thromboplastin; PT, prothrombin time; HIT, heparin-induced thrombocytopenia; PCI, percutaneous coronary intervention; ACT, activated clotting time; PTCA, percutaneous transluminal coronary angioplasty.
Major Clinical Trials
Thrombin Inhibitors Trialist (DTIT) Collaborative Group.31 This meta-analysis reviewed the use of direct thrombin inhibitors in acute coronary syndrome (ACS). A total of 5,674 patients were included, of whom 4,603 underwent PCI. Bivalirudin reduced the risk of death and MI by about 30% in the few weeks after the procedure compared to heparin.
HERO-2.32 This trial compared bivalirudin to heparin in patients receiving streptokinase therapy for acute MI. The conclusion of the study was that the use of bivalirudin was associated with reduction of reinfarction rates within 96 hours, but it was not associated with reduction in mortality. It was associated with a slight increase in the risk of bleeding.
HASI.33 This trial showed that bivalirudin is as effective as heparin in preventing ischemic complications in UA or after PCI. It was found to be superior to heparin in reducing immediate post-MI complications. In this study, bivalirudin was associated with increased incidence of bleeding. In an update of the study that was published,34 bivalirudin was better than heparin in reducing death, MI, revascularization, and bleeding (as a combined endpoint) in patients with UA or post infarction angina who were undergoing PCI. The difference stemmed mainly from a reduction in the rate of revascularization.
OASIS.35 This study compared lepirudin to heparin in ACS. Lepirudin reduced mortality and reinfarction at 3 days after the event compared to heparin but was associated with an increased cost and risk of major bleeding, which required transfusion.
REPLACE-II.36 In this trial, bivalirudin, given during PCI as an IV bolus followed by an infusion, was compared to heparin and glycoprotein (GP) IIb/IIIa inhibitors given 12 to 18 hours after the procedure. The study was set to assess for noninferiority for primary endpoint of death, MI, urgent revascularization, or major bleeding. Bivalirudin was found to be associated with a significant reduction in major bleeding compared to heparin and GP IIb/IIIa inhibitor.
RE-LY.37 This study compared warfarin to dabigatran 150 mg twice daily and dabigatran 110 mg twice daily in patients with non-valvular atrial fibrillation and at least one additional risk factor for stroke. The primary outcome was stroke or systemic embolism. Dabigatran in both doses were found to be noninferior to warfarin for primary outcome, with the 150 mg dabigatran dose superior to warfarin with similar rates of bleeding.
ACUITY.38 In this trial, patients with ACS undergoing early invasive strategy were treated with one of three regimens: UFH or enoxaparin plus a GP IIb/IIIa inhibitor, bivalirudin plus a GP IIb/IIIa inhibitor, or bivalirudin alone. Primary end-points included a composite ischemia endpoint (death, MI, and unplanned revascularization for ischemia), as well as major bleeding, and the combination of composite ischemia or major bleeding. Bivalirudin plus GP IIb/IIIa inhibitor compared to heparin plus IIb/IIIa inhibitor was found to be non-inferior for the composite ischemia endpoint as well as for major bleeding. Bivalirudin alone was found to have significantly reduced rates of major bleeding and was noninferior compared to heparin plus GP IIb/IIIa inhibitor for ischemic events.
HORIZONS-AMI.39 This trial compared the use of bivalirudin to heparin plus GP IIb/IIIa inhibitor in patients with STEMI who presented within 12 hours of symptoms and were to undergo primary PCI. Major bleeding and combined adverse cardiovascular events (combination of major bleeding or major adverse cardiovascular events, including death, reinfarction, target-vessel revascularization for ischemia, and stroke) within 30 days were the two primary endpoints. The rate of net adverse clinical events with bivalirudin alone compared to heparin plus IIb/IIIa inhibitor was reduced (9.2% vs. 12.1%; RR, 0.76; 95% CI, 0.63 to 0.92; p = 0.005). Major bleeding was reduced with bivalirudin use (4.9% vs. 8.3%; RR, 0.6; 95% CI, 0.46 to 0.77; p <0.001). Rates of major adverse cardiac events were similar between the groups. The 30-day rate of death from cardiac causes was also reduced with bivalirudin (1.8% vs. 2.9%; RR, 0.66; 95% CI, 0.40 to 0.95; p = 0.03). The rate of stent thrombosis in the first 24 hours occurred more in the bivalirudin group (1.3% vs. 0.3%, p < 0.001). However, the 30-day rate of stent thrombosis was similar between the groups (2.5% vs. 1.9%, p = 0.30).
Low-Molecular-Weight Heparin: Dalteparin (Fragmin), Enoxaparin (Lovenox), Tinzaparin (Innohep)
Mechanism of Action
Each of these three drugs binds to antithrombin, increasing antithrombin’s activity.
Side Effects
Common side effects include bleeding and thrombocytopenia (type II occurs less often than with UFH but is still a concern), rash, hematoma at the injection site, and fever.
Indications and Precautions
Dalteparin is indicated for prophylaxis of DVT in high-risk patients undergoing abdominal surgery, hip replacement surgery, and patients who are immobile during acute illness. Dalteparin is also indicated in UA/non–Q-wave myocardial infarction (NQWMI) for the prevention of ischemic complications in patients on concurrent aspirin therapy. It should be avoided in patients with HIT or suspected HIT. Although there is a lower reported incidence of thrombocytopenia with LMWH than with UFH, it can still occur. Cross-reactivity of patients developing HIT type II on UFH to those receiving LMWH is >90%. Because it is cleared renally, it is contraindicated in patients with severe renal insufficiency (creatinine clearance <30 mL/min).
Enoxaparin is labeled for prevention of DVT in patients undergoing hip replacement surgery, during and following hospitalization for knee replacement surgery, or in patients undergoing abdominal surgery who are at risk for thromboembolic complications, as well as in medical patients who are at risk for thromboembolic complications due to severely restricted mobility during acute illness. Other indications include the prevention of ischemic complications of UA and non–ST elevation and ST-elevation MIs when coadministered with aspirin. Furthermore, it is labeled for use with warfarin for inpatient treatment of acute DVT ± PE or outpatient treatment of acute DVT without PE when administered in conjunction with warfarin. Enoxaparin is renally cleared, dose reductions are required for creatinine clearance < 30 mL/min, and it has not been approved for use for patients on dialysis.
Tinzaparin is labeled for treatment of acute symptomatic DVT with or without PE when administered in conjunction with warfarin sodium. The safety and effectiveness of tinzaparin were established in hospitalized patients. The same precautions apply as for other LMWH products.
Major Clinical Trials
ESSENCE.40 In this trial, IV UFH was compared with enoxaparin in patients with angina at rest or NQWMI. The primary end-point, the composite of risk of death, (MI, or recurrent angina at 14 days was reduced with enoxaparin by 16.2% (OR 0.8; 95% CI, 0.67 to 0.96; p = 0.02). Secondary endpoints that included the triple composite at 30 days were reduced with enoxaparin by 15% (OR 0.81; 95% CI, 0.68 to 0.96; p = 0.02). However, individual endpoints of death or MI were not significant at 14 or 30 days. Only 16.7% of patients received PCI. No difference was found in major hemorrhage or hemorrhagic stroke, although a higher risk of hemorrhage overall was found with enoxaparin versus heparin (18.4% vs. 14.2%, p = 0.001) due to the increase in minor hemorrhage at 30 days.
A to Z Trial.41 The A to Z trial compared enoxaparin to IV UFH with concomitant tirofiban and aspirin in patients with Non–ST-elevation acute coronary syndrome (NSTE-ACS). The primary endpoint composite of all-cause death, MI, or refractory ischemia for enoxaparin was 8.4% compared to UFH that was 9.4% (HR 0.88, 95% CI, 0.71 to 1.08), which met criteria for noninferiority. The rate for thrombosis in myocardial infarction (TIMI) grade bleeding was reported in 3.0% of enoxaparin patients (vs. 2.2% for UFH) p =0.13.
ASSENT-3.42 The safety and efficacy of tenecteplase in combination with enoxaparin, abciximab, or UFH were studied in patients with AMI. Patients received full-dose tenecteplase with enoxaparin, half-dose tenecteplase with low-dose weight-adjusted UFH and abciximab for 12 hours, or full-dose tenect-eplase with weight-adjusted UFH for 48 hours. The primary efficacy endpoint was the composite of mortality, in-hospital reinfarction, or in-hospital refractory ischemia at 30 days. The addition of in-hospital intracranial hemorrhage or in-hospital major bleeding other than intracranial bleed made up the primary plus safety endpoint. The primary endpoint for the enoxaparin, abciximab, and UFH groups were (11.4%, 11.1%, and 15.4%; p = 0.001). Enoxaparin may have performed better due to the 7-day treatment as opposed to the 48 hours of UFH. The primary efficacy and safety endpoints were enoxaparin (13.8%), abciximab (14.2%), and UFH (17.0%), p = 0.0081. There was no difference found between the groups for 30-day mortality. A significant difference was found in major bleeding other than intracranial hemorrhage with enoxaparin (3.0%), abciximab (4.3%), and UFH (2.2%), p = 0.0005. The bleeding was not found to be significantly different between the enoxaparin and UFH groups. However, the abciximab group, specifically, was found to have an increase in major bleeding compared to the UFH group, especially in patients >75 years (13.3% vs. 4.1%) and in patients with diabetes (7.0% vs. 2.2%).
EXTRACT.43 Enoxaparin was compared to UFH as adjunct therapy with fibrinolytic therapy in patients with STEMI. With fibrinolytic therapy, patients received either UFH for at least 48 hours or enoxaparin. Enoxaparin was continued through the hospital stay, or a maximum of 8 days. Primary endpoint was composite of death from any cause, or nonfatal recurrent MI through 30 days that was 9.9% for enoxaparin and 12.0% for UFH (0.83 RR; 95% CI, 0.77 to 0.90, p < 0.001). There was no difference in death between enoxaparin (6.9%) and UFH (7.5%) (p = 0.11). There was a significant increase in the rate of bleeding with enoxaparin (2.1%) compared to 1.4% for UFH (1.53 RR; 95% CI, 1.23 to 1.89, p < 0.001).
SYNERGY.44 Enoxaparin was compared to UFH for efficacy and safety in high-risk patients with non–ST-segment elevation ACS managed with an early invasive strategy. Primary endpoint of death or nonfatal MI by 30 days was 14.0% (696/4,993) in the enoxaparin group and 14.5% (722/4,985) in the UFH group (HR, 0.96; 95% CI, 0.86 to 1.06). Enoxaparin was not superior but did meet noninferiority criteria. Enoxaparin had an increase in TIMI major bleeding (9.1% vs. 7.6%, p = 0.008), but not in GUSTO (Global Utilization of Streptokinase and t-Pa for Occluded Arteries) severe bleeding (2.7% vs. 2.2%).
This class of drugs is chemically and pharmacologically diverse. Before we classify the drugs, we will review some information on the various calcium channels. The main ports of entry for calcium into the cell are via voltagegated calcium channels, which open when the membrane depolarizes. Another is a sodium–calcium channel, which moves one calcium ion out in exchange for three sodium ions entering into the cell. The electrical balance within a cell is accomplished by the sodium/potassium ATP channel. Calcium is normally stored in the sarcoplasmic reticulum, which also helps control the level of intracellular calcium. There are three types of calcium channels, L, N, and T. They differ in distribution in various tissues, duration of opening, and voltage range. Only L and T are of interest in cardiology.
L-Type Channels
L-type channels are found in heart muscle and in parts of the conducting system, smooth muscle, brain, adrenals, and kidneys. Although all calcium channel blockers bind to the calcium channel receptor, they have different binding sites. Blocking the L channels or receptors inhibits inward calcium currents into the cell, thus reducing the concentration of calcium needed for muscle contraction, which leads to smooth muscle dilation, decreasing contractility of heart muscle, slowing of the sinoatrial (SA) node firing rate, and increasing AV nodal conductance time.
T-Type Channels
T-type channels are found in blood vessels, adrenals, brain, kidneys, the heart conduction system, and in heart muscle under pathologic conditions such as cardiomyopathy. There is only one identified binding spot for medications, and blocking this receptor leads to dilation of peripheral and coronary blood vessels, thus decreasing SVR and increasing myocardial blood flow. Also, blockade of T-type channels produces a lowering of heart rate.
Mechanism of Action
The various classes of calcium channel blockers and the drugs in each class, as well as their mechanism of action, are shown in Table 61.7.
TABLE
61.7 Calcium Channel Blockers
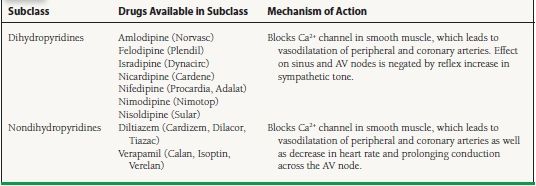
AV, atrioventricular.