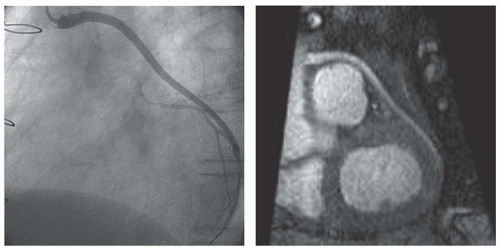
Flow through a blood vessel may or may not be impaired by a stenosis, visualized by angiography (
19,
20). The assessment of blood flow through coronary arteries and bypass grafts has gained wide attention. To evaluate the hemodynamic impairment of a lesion, flow is measured at rest and during pharmacologically induced stress with adenosine or dipyridamole (
21). By dividing the flow value during stress by the flow value at rest, the coronary flow reserve (CFR) is calculated (
22,
23). Flow-limiting stenoses cause a compensatory vasodilation at rest to maintain sufficient blood flow to the myocardium. As a consequence, the blood vessel cannot respond adequately to an increase in absolute flow by vasodilation during stress and CFR will be reduced. At first, blood flow was determined by Doppler flow transducers at open-chest procedures (
19,
22,
23,
24,
25 and
26), limiting extensive use of CFR in clinical practice. When the diameter of intravascular catheter-based Doppler ultrasonographic devices could be reduced to 0.018 in., it became feasible to measure the velocity of the blood flow and calculate the coronary flow velocity reserve (CFVR) for coronary arteries in patients during catheterization. Blood flow correlated well with Doppler-derived velocity of blood flow both in vitro and in vivo (
27,
28 and
29). Invasive Doppler-derived CFVR has proven its potential in numerous clinical applications, such as in identifying hemodynamic significant stenoses in native coronary arteries and vein grafts (
30,
31), in the functional assessment of stenoses of intermediate severity (
32), in the determination of the need for and the outcome after coronary intervention (
33,
34 and
35), and in the prediction of restenosis (
36).
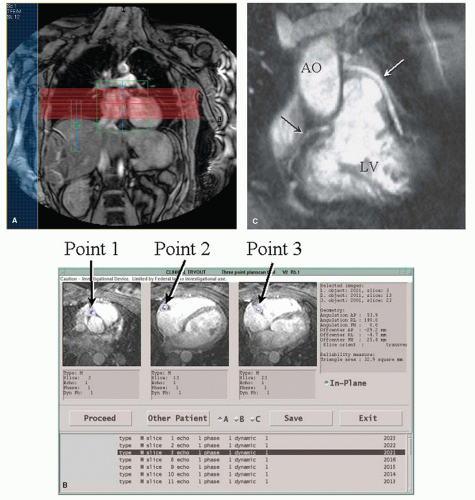
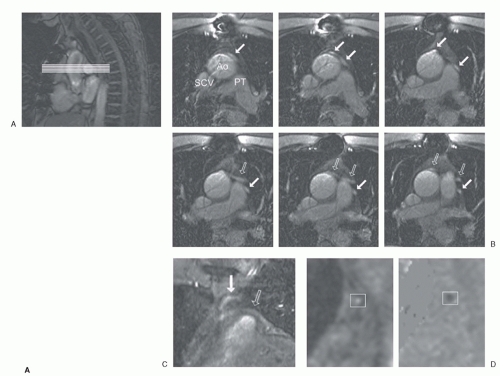
With the use of CMR, blood flow velocity can be measured using phase-contrast, velocity-encoded sequences (
37). To use such a sequence accurately, an imaging plane is planned perpendicular to the target vessel. Throughout the cardiac cycle, the acquisition yields anatomic modulus images paired with phase images, in which every pixel contains a different velocity value. Volume flow (mL/min) can be obtained by calculating the integrated volumetric flow rate of all pixels in the vessel lumen per heartbeat and multiply it with the heart rate. To display the flow pattern, a flow rate-versus-time graph is drawn. Alternatively, the central peak velocity (cm/sec) can be obtained by selecting several pixels in the vessel center.
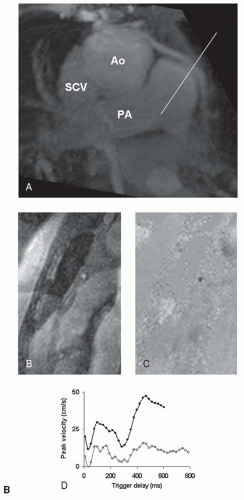
Figure 23.4 depicts a typical example of a CMR examination with velocity mapping in a vein graft.
Early Magnetic Resonance Studies in Vein Grafts
For vein grafts, feasibility to quantify flow and characterize the flow pattern noninvasively by CMR was demonstrated (
38,
39). In an early study graft flow <20 mL/min and a loss of the biphasic flow pattern, typical for bypass grafts, would indicate a dysfunctional graft (
40). In another
study adequate biphasic flow profiles could be obtained in 62 of 73 angiographically patent grafts (
8). A significant difference in flow between single and sequential grafts to three vascular regions was demonstrated. These early vein graft flow studies were limited by the use of a gradient-echo sequence with limited spatial resolution (1.9 × 1.2 × 5 mm
3) and no compensation for respiratory motion on 0.5- to 0.6-T magnets.
Early Magnetic Resonance Studies in Arterial Grafts
Feasibility to quantify flow in native and grafted internal mammary arteries (IMAs) was demonstrated using a free-breathing gradient-echo sequence on a 1.5-T MR scanner (
41). A large intersubject variation of IMA graft flow (range: 28 to 164 mL/min) was observed. Mean flow and peak velocity were lower in IMA grafts compared with native IMAs. In a different feasibility study, respiratory motion was compensated using a breath-hold, segmented k-space, gradient-echo sequence to quantify velocity in native and grafted IMAs (
42). Comparison with a free-breathing technique in native IMAs demonstrated a higher peak velocity using the breath-hold sequence because of elimination of respiratory motion artifacts and averaging of velocities. This sequence allowed imaging time to decrease from approximately 4 minutes at free breathing to a 20-second breath-hold. By means of a view-sharing reconstruction, an effective temporal resolution of 64 milliseconds could be obtained, allowing an acquisition of 7 to 13 temporal phases per cardiac cycle.
Angiographically Controlled Magnetic Resonance Studies in Vein and Arterial Grafts
To evaluate the diagnostic value of MR flow velocity in bypass grafts, several angiographically controlled studies were performed. With the use of breath-hold sequences, flow measurements at rest and during pharmacologically induced stress with determination of CFR in bypass grafts were achieved. By the use of a breath-hold turbo-field echo-planar imaging sequence, a significant increase at stress for flow and velocity parameters was observed and CFR (mean 2.7 ± 1.1 for single grafts) could be calculated (
43). A significant difference between grafts with <50% and >50% stenosis was demonstrated for MR-derived average and diastolic peak velocity during stress (
44).
The detection of >70% angiographic stenosis in IMA grafts by breath-hold MR flow velocity at rest and during stress was investigated (
45). At CABG surgery, titanium clips were used to avoid metal artifacts at MR imaging. A significant difference between grafts with <70% and >70% stenosis was demonstrated for baseline mean blood flow and the diastolic-to-systolic velocity ratio. Threshold values of 35 mL/min for baseline mean flow and 1 for diastolic-to-systolic velocity ratio were proposed to separate IMA grafts with <70% and >70% stenosis with respective sensitivity and specificity of 86% and 94%, and 86% and 88%. CFR did not differ significantly between IMA grafts with <70% and >70% stenosis.
The value of MR flow in the prediction of vein graft disease was assessed (
46). An algorithm was formulated combining baseline flow <20 mL/min or CFR <2 to detect grafts or run-offs with a significant stenosis (>50%) or a myocardial infarction in the graft vascular territory, yielding a sensitivity of 78% with a specificity of 80%. The algorithm was designed to exclude normal-functioning vein grafts from further invasive examinations. A different approach for the detection of stenotic vein and arterial grafts or recipient vessels by MR with velocity mapping was also formulated, in which single and sequential vein and arterial grafts were separately analyzed (
47). Sensitivity and specificity for detecting single vein grafts with >70% stenosis were 96% and 92%, respectively. For sequential vein grafts sensitivity and specificity were 94% and 71%, respectively. A proposed cut-off point for separating <70% and >70% stenosis in single vein grafts was 1.43 for CFVR. Not enough stenoses in arterial grafts were present to formulate an adequate model.
The detection of graft disease was further improved by a combined approach with MRA and MR flow measurements, which showed a sensitivity and specificity of 95.2% and 96.8% for detecting significant stenoses (>50%) in IMA grafts and 100% and 97.8% in vein grafts (
48).
These studies show that MR flow velocity assessment has potential to become a diagnostic tool in predicting patency and the presence or absence of significant stenosis in both vein and IMA grafts in daily practice. However, only if a full examination of all grafts and native coronary arteries in a single MR imaging session is feasible, then may this technique reach its full value.