17.3.2 Clinical features
Key features
- Acute shortness of breath: this is the most common presenting symptom and is often severe
- Cough productive of pink, frothy sputum
- Patient appears acutely unwell: sweaty, anxious and fatigued.
Examination findings
- There is often a combination of fluid overload and a low cardiac output state
- Respiratory distress: tachypnoea, pallor, cyanosis
- Fluid overload:
- raised JVP
- peripheral oedema
- S3 gallop rhythm
- lung crepitations: key diagnostic feature, often inspiratory and bibasal
- pleural effusions: stony dull percussion at lung bases
- raised JVP
- Low cardiac output state:
- tachycardia
- hypotension, although hypertension is more common in the acute setting as a consequence of high circulating blood volumes and catecholamines secondary to the stress of illness
- cool peripheries with a slow capillary refill time
- oliguria.
- tachycardia
EXAM | Shock (SBP <90 mmHg) is concerning in an acute heart failure patient, and urgent intervention is required. |
17.3.3 Investigations
APO will likely be apparent after clinical history-taking and examination. Investigations aim to identify the aetiology, confirm the diagnosis and determine the baseline function of the patient.
First-line
- Blood tests: FBC, U&Es, WCC, TFTs, troponin (only if ACS suspected/needs to be excluded).
- ECG: looking for evidence of acute MI (NSTEMI or STEMI), arrhythmia, heart block, etc.
- CXR: the most important acute investigation. It may show interstitial oedema, alveolar ‘batwing’ oedema, upper lobe venous diversion and bilateral pleural effusions.
- Echocardiography is an important investigation in cardiogenic APO. It may show left ventricular systolic dysfunction and valvular abnormalities. However, it is not always abnormal, especially in non-cardiogenic APO.
- Arterial blood gas: PaO2 is often low and may guide oxygen therapy; monitoring the PaCO2 is important too, as patients are at risk of developing type II respiratory failure.
- Urinary catheterisation: to help monitor fluid balance and response to diuretics (e.g. measuring hourly urine output).
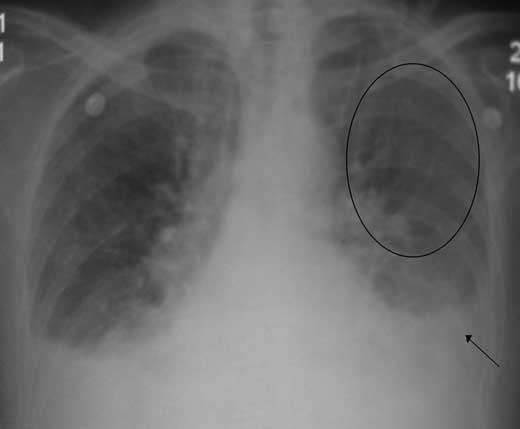
Figure 17.1 – Chest radiograph illustrating vascular redistribution (circled) and bilateral pleural effusions (arrow).
Second-line
- Brain natriuretic peptide (BNP): high sensitivity and specificity for LV dysfunction. However, the diagnosis is usually clinical and measurement is not routine.
- Cardiac catheterisation: may be utilised in determining haemodynamic stability and to detect coronary artery disease.
- Invasive haemodynamic monitoring such as central venous pressure monitoring is useful in severe cases where it is able to guide filling pressures, determine cardiac output and assess response to therapy.
17.3.4 Management
The aims are to optimise myocardial oxygenation and safely reduce preload and afterload.
- Sit the patient up: this reduces anxiety and the work of breathing
- 15 L/min oxygen
- IV morphine: reduces anxiety and dyspnoea, venodilates (reduces afterload) and antagonises the effects of catecholamines, thereby reducing myocardial oxygen demand
- Nitrates: should only be given if SBP >100 mmHg
// Why? //
GTN is a venodilator that reduces afterload and preload, and thus cardiac work. An infusion should be started but a drop in blood pressure is usually a consequence.
- IV furosemide: venodilates (immediate effect) and diuresis (delayed effect).
If there is failure of the above or cardiogenic shock:
- Refer for more advanced care: coronary care unit (CCU) or intensive care unit (ICU).
- May require inotropic support such as dobutamine if hypotensive. Inotropes have, however, been associated with increased mortality due to risk of myocardial ischaemia.
- Non-invasive ventilation: continuous positive airway pressure is indicated if there is failure to maintain oxygenation or the patient develops type II respiratory failure, despite above therapy. It improves survival and reduces need for endotracheal intubation.
17.4 Cardiac tamponade
Cardiac tamponade In A Heartbeat
Epidemiology | 0.2 per 100 000 |
Aetiology | Seen frequently with pericarditis. Trauma is an important cause in the young. |
Clinical features | Can present acutely with dyspnoea and shock. Subacutely with oedema and fatigue. |
Investigations | ECG and CXR may aid in diagnosis. Echocardiography is investigation of choice. |
Management | Prompt pericardiocentesis or thoracotomy are required depending on severity and cause. |
Cardiac tamponade refers to the haemodynamic decompensation that occurs secondary to fluid or air in the pericardial space. The resulting high intra-pericardial space pressures prevent the heart from expanding during diastolic filling, reducing cardiac output. Congestion eventually impedes venous return resulting in some of the classic features of right heart overload. The condition is rapidly fatal (in the acute form) and depends on a clinical diagnosis.
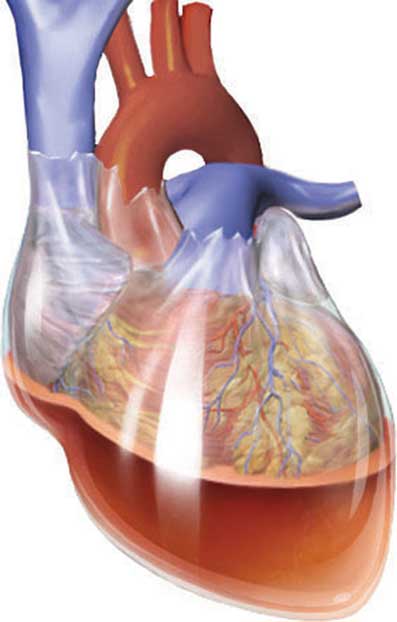
Figure 17.2 – An illustration of blood in the pericardial space.
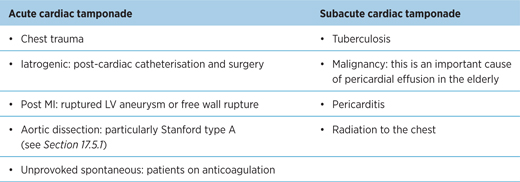
17.4.1 Aetiology
Table 17.2 – Causes of acute and subacute cardiac tamponade
17.4.2 Clinical features
Key features
- The hallmark features are dizziness and collapse, and reflect hypotension
- It also commonly presents acutely with dyspnoea and non-exertional chest pain
- Subacute: fatigue, altered mental status
- It may present as shock or cardiac arrest.
Examination findings
EXAM | Beck’s triad: 3Ds
|
- Tachycardia
- Kussmaul’s sign: rise of JVP with inspiration
- Pulsus paradoxus: SBP rise of >10 mmHg on inspiration (refer to Chapter 6)
// PRO-TIP //
Hypotension and a raised JVP should alert you to several diagnoses: cardiac tamponade, constrictive pericarditis, tension pneumothorax and pulmonary embolism.
17.4.3 Investigations
The diagnosis of cardiac tamponade requires a high index of clinical suspicion. The aims of investigations are to identify the baseline function of the patient, confirm the diagnosis and identify the underlying aetiology.
First-line
- URGENT echocardiography to assess the size and distribution of the pericardial effusion and presence of any indicators of haemodynamic compromise (e.g. RA/RV free wall collapse in diastole)
- Blood tests: FBC, U&Es, CRP, TFTs
- ECG: low-voltage QRS complexes and sinus tachycardia. Electrical alternans is where there is a variation in the amplitude of the QRS complex and indicates the presence of a large effusion

Figure 17.3 – Electrical alternans: ECG tracing showing low-voltage, alternating QRS morphology.
- CXR: globular cardiomegaly. The size of the heart does not correlate with haemodynamic stability. Conversely, the heart may not be enlarged.
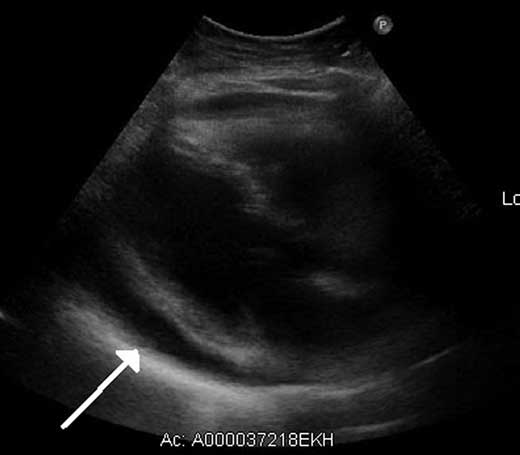
Figure 17.4 – An echocardiograph illustrating fluid in the pericardial space (arrow).
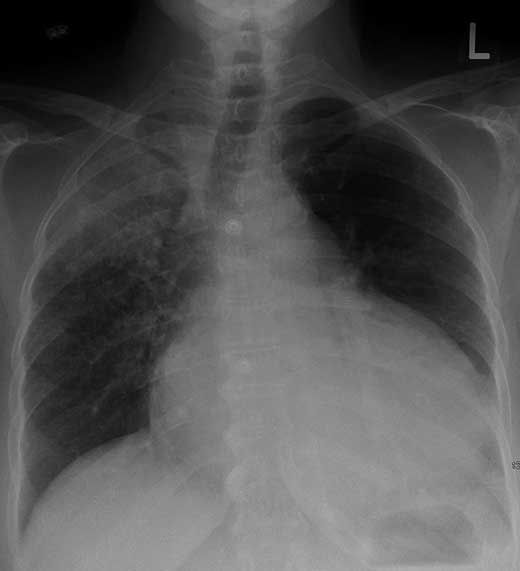
Figure 17.5 – Large pericardial effusion secondary to malignancy. Note primary lung lesion in right upper lobe.
17.4.4 Management
- Leg elevation and bed rest to improve venous return
- IV fluid resuscitation – may require colloid and inotropic support
- Urgent draining with echocardiography-guided pericardiocentesis if patient is demonstrating signs of cardiac tamponade; semi-elective drainage is preferred in less urgent cases
- Urgent thoracotomy if secondary to trauma
- Pericardial fluid aspirate should be sent for microbiology (+TB) and cytology to identify a potential underlying aetiology
- Pericardiocentesis should be performed with caution in patients with:
- moderate to severe pulmonary hypertension, as drainage of the pericardial fluid may reduce right ventricular support, leading to worsening RV function
- coagulopathies. The risk of bleeding is higher in patients with coagulopathies, and the loss of blood may prove to be life-threatening.
- moderate to severe pulmonary hypertension, as drainage of the pericardial fluid may reduce right ventricular support, leading to worsening RV function
// PRO-TIP //
It is important not to delay pericardiocentesis for imaging if the patient is haemodynamically compromised.
17.5 Aortic dissection
Aortic dissection In A Heartbeat
Epidemiology | 3 per 100 000. More common in males, affecting those aged 50–70 years. |
Aetiology | Most commonly due to hypertension. |
Clinical features | Severe tearing chest/back pain in a patient with hypertension should prompt exclusion of aortic dissection. |
Investigations | Blood tests, ECG, CXR and TTE are 1st-line. CT aortogram is diagnostic and important for management. |
Management | BP control is vital, with a target of <120 mmHg. Surgery if type A or end-organ damage. |
An aortic dissection (AD) refers to a tear in the tunica intima of the aorta that results in blood entering the space between the tunica media and adventitia. The space extends across the length of the vessel as the blood pressure forces dissection. The most commonly affected areas are the proximal ascending aorta or the aorta just distal to the origin of the subclavian vessel. The dissection may rupture, and if type A, will result in cardiac tamponade and rapid death.
17.5.1 Stanford classification
- Type A: dissection of the ascending aorta. This is a surgical emergency.
- Type B: dissection sparing the ascending aorta. Medical management takes precedence unless there is evidence of end-organ damage.
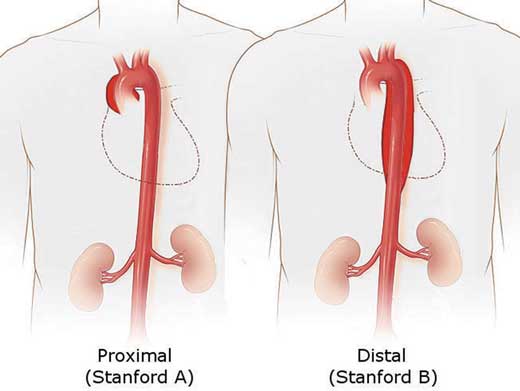
Figure 17.6 – Stanford classification of aortic dissection.
17.5.2 Aetiology
- Hypertension (~75%)
- Bicuspid aortic valve and aortic regurgitation (14–30%)
- Connective tissue disorders – Marfan’s syndrome: dissection is most common cause of death; Ehlers–Danlos type IV
- Iatrogenic: angiography
17.5.3 Clinical features
Key features
- Severe, instant onset of tearing or pulsating anterior chest (ascending) or interscapular pain (descending) that goes down the back as the dissection progresses.
- Chest pain is often the only symptom present. It must be suspected in anyone presenting with severe chest pain even though it is a relatively uncommon diagnosis.
- Syncope in 10–20%.
Examination findings
- Blood pressure discrepancy: different BPs in both arms is a classic feature
- Radio–radio delay
- Weak femoral pulses that often disappear and reappear
- Signs of underlying condition
- hypertension: more common than hypotension despite the appearance of a shocked patient
- aortic regurgitation.
- hypertension: more common than hypotension despite the appearance of a shocked patient
17.5.4 Investigations
First-line
Immediate investigations
- Blood tests: FBC, U&Es, group and save or cross-match: depending on level of haemodynamic compromise. D-dimer testing (values <500 ng/ml) is a useful screening tool for identifying those patients who do not have aortic dissection: i.e. it has a high negative predictive value (96% in some studies).
- ECG: main aim is to exclude acute coronary syndrome. The right coronary artery is most commonly affected in type A aortic dissection and may manifest as myocardial ischaemia, NSTEMI or STEMI, depending on the degree of occlusion. Less commonly, heart block may also ensue.
- CXR: typical features include a widened mediastinum in 90% and a left pleural effusion in descending dissections, but the CXR may be normal. It may exclude other pathology.
- Transthoracic echocardiography: for rapid assessment of an ascending aortic dissection, but low sensitivity limits its usefulness. It may be diagnostic but patients require further imaging to determine the extent of dissection.
Advanced investigations
- CT aortogram is the investigation of choice with a sensitivity and specificity of >96%. It is diagnostic and will also allow categorisation and selection of further treatment.
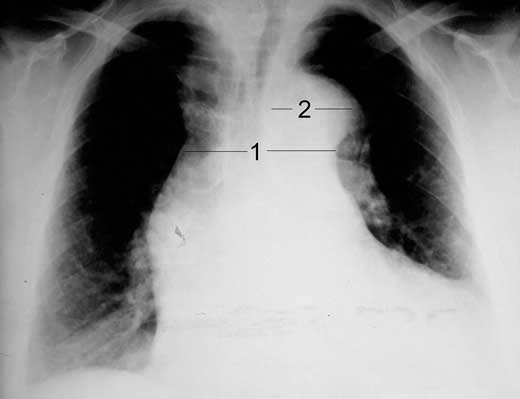
Figure 17.7 – Widened mediastinum of aortic dissection Stanford type A.
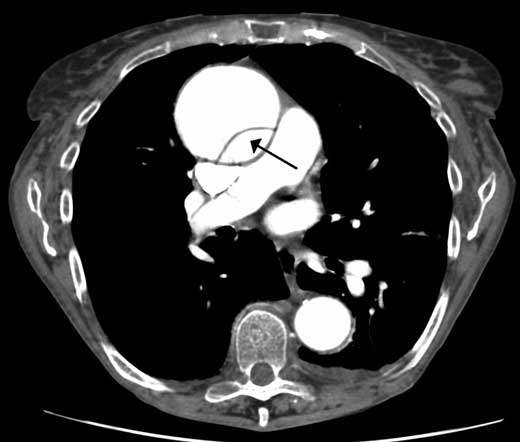
Figure 17.8 – A dissection of the ascending aorta on a CT aortogram.
Second-line
- MRI has a 99% sensitivity but is often unsuitable in the unstable patient
- Transoesophageal echocardiography: for proximal ascending aortic dissection.
17.5.5 Management
Immediate management
- Give oxygen
- Wide bore cannula access: in case of circulatory collapse, otherwise do not give IV fluids
- Analgesia.
EXAM | BP control: aim for an SBP of <120 mmHg; a range from 100–120 mmHg is ideal. This may require the use of antihypertensive IV infusions, which include labetalol and GTN. Rigorous BP control is key in the management of aortic dissection and is one of the few examples where the blood pressure is rapidly reduced if the patient were suffering from accelerated/malignant hypertension (see below). |
// PRO-TIP //
Further management
- Type A: should be treated surgically as an emergency – open replacement or endovascular stent repair.
- Type B: medical conservative management is the mainstay with tight BP control.
- Long-term management of hypertension is required to prevent further events, with close surveillance. Patients may require endovascular stenting if aortic enlargement is present.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


