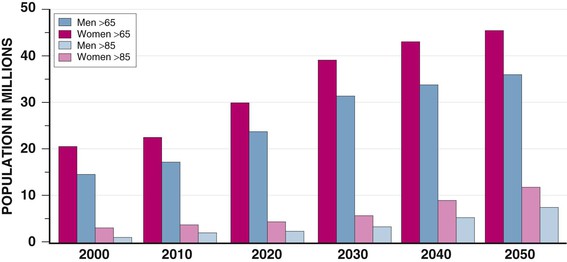
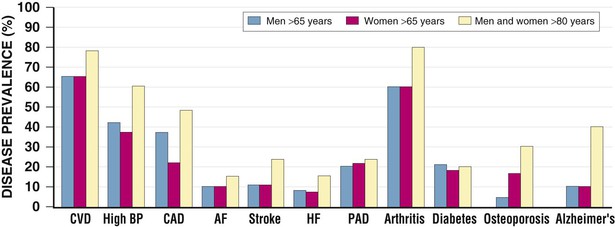
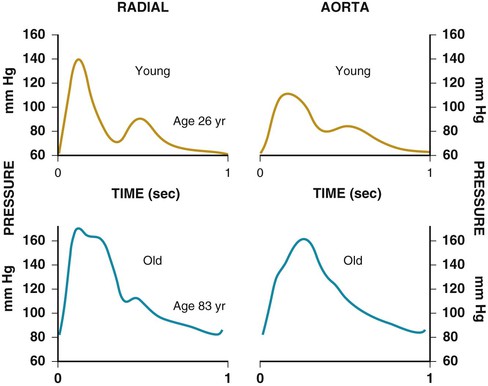
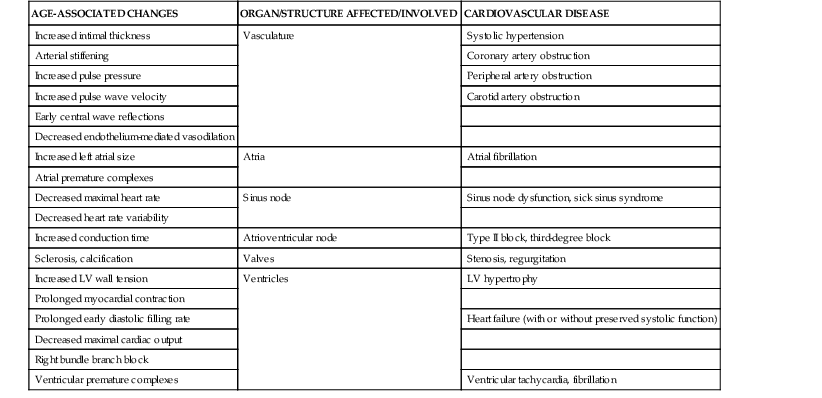
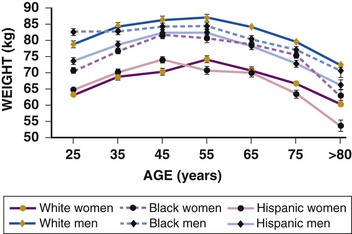
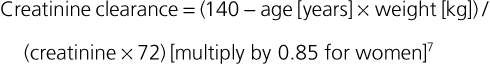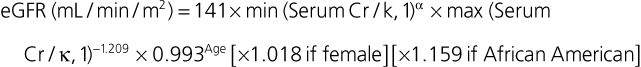Janice B. Schwartz, Douglas P. Zipes Cardiovascular disease is both the most frequent diagnosis and the leading cause of death among both men and women older than 65 years. Hypertension occurs in one half to two thirds of people older than 65 years, and heart failure (HF) is the most frequent hospital discharge diagnosis among older Americans. The profile of these common cardiovascular diseases differs in older patients from that in younger patients. Systolic but not diastolic blood pressure increases with aging, resulting in increased pulse pressure. Systolic hypertension becomes a stronger predictor of cardiovascular events, especially in women (see Chapter 43). HF with preserved ejection fraction becomes more common at older ages and is more common in women (see Chapter 27). Coronary artery disease (CAD) is more likely to involve multiple vessels and to affect the left main artery and occurs with similar frequency in women and in men older than 65 years. Equal numbers of older men and women present with acute myocardial infarction (MI) until the age of 80 years, after which this presentation is more common in women. Non–ST-segment rather than ST-segment elevation MI accounts for two thirds of the cases in older patients (see Chapters 51 to 54). Diagnosis of stroke is made in 20% to 25% of patients after the age of 80 years (see Chapter 59). More than 80% of all deaths attributable to cardiovascular disease occur in people older than 65 years, with approximately 60% of deaths in those older than 75 years. The high morbidity and mortality from cardiovascular disease in the elderly population warrant aggressive approaches to prevention and treatment that are effective in older patients. Compelling data demonstrate reduced morbidity and mortality rates for the treatment of hypertension, HF with reduced ejection fraction, atrial fibrillation, acute coronary syndromes, CAD, stroke, diabetes, and lipid abnormalities in older patients 60 to 74 years of age, although data on minorities and women are limited. Fewer trials of cardiovascular therapies have enrolled significant numbers of men or women older than 75 years, elderly patients with multisystem disease, or elderly patients with cognitive impairment, and none have addressed cardiovascular therapies in the nursing home population. When clinical trials enroll older patients, participants differ markedly from the most older patients. The projected increase in numbers of older people from previously understudied and undertreated groups presents both medical and economic challenges for cardiovascular disease treatment. No universal definition of “elderly” has been recognized, nor has an accurate biomarker for aging been identified. Although physiologic changes associated with aging do not appear at a specific age and do not proceed at the same pace in all people, most definitions are based on chronologic age. The World Health Organization uses 60 years of age to define “elderly,” whereas most U.S. classifications use the age of 65 years. Gerontologists subclassify the older population into three age groups: young old (60 to 74 years), old old (75 to 85 years), and very old (older than 85 years of age). Cardiovascular society statements have addressed differences in responses between patients older than 65 years, those 65 to 74 years, and those 75 to 84 years of age separately from those older than 85 years of age. Clinicians often separate older patients into two subgroups—those 65 to 80 years of age and those older than 80 years, to highlight the frailty, reduced capacity (physical and mental), and presence of multiple disorders that are more common after the age of 80. The vast majority of therapeutic interventions for elderly patients are pharmacologic, making appropriate drug selection and modification of dosing regimens for this population important (see Chapter 9). On average, body size decreases with aging, and body composition changes result in decreased total body water, intravascular volume, and muscle mass. Age-related changes are continuous but are most pronounced after the age of 75 to 80 years. Women, with the exception of African American women, tend to weigh less and have smaller intravascular volumes and muscle mass than men at all ages (Fig. 76-4). Higher serum concentrations of medications will be found in older patients, and especially older white and Asian women, if initial doses are the same as in younger patients. Weight adjustments for loading doses of the cardiovascular drugs digoxin and type I and type III antiarrhythmic drugs, aminoglycoside antibiotics, chemotherapy regimens, and unfractionated heparin are standard (see Chapter 7). When fibrinolytic drugs have been administered without weight-based dosage adjustments, increased risk of intracranial hemorrhage has been documented in older, smaller, and female patients.6 Bleeding associated with low-molecular-weight heparins in combination with other lytic agents can be reduced by using weight-based dosing. Routine weight-based adjustments should be made in loading doses of medications, especially those with a narrow therapeutic index. The result usually is a lower loading dose in older patients than in younger patients, with the lowest doses in older white and Asian women. Hepatic drug clearance is the net result of processes that include drug delivery to the liver (by portal vein and hepatic artery blood flow) and enzymatic transport into liver cells and enzymatic biotransformation and/or excretion via efflux transporter enzymes into the bile. These processes are influenced by heterogeneous factors that include genetic, disease, and environmental influences and metabolic enzymes that can be both inhibited and induced (see Chapter 9, Table 9-2), contributing to the lack of clinically validated algorithms to estimate hepatic and extrahepatic drug clearance or potential age-related changes. Drugs metabolized by the phase II conjugative reactions of glucuronidation (morphine, diazepam), sulfation (methyldopa), or acetylation (procainamide) do not appear to be affected by aging but show disease-related effects, may show frailty-related decreases, and show consistently lower clearance in women compared to men. In vitro, age-related changes are seen in phase I oxidative biotransformation by the membrane bound cytochrome P-450 (CYP) oxidative group of enzymes responsible for elimination of more than 50% of metabolized drugs. Cardiovascular drugs showing age-related decreases in CYP-mediated clearance in human investigations include alpha blockers (doxazosin, prazosin, terazosin), some beta blockers (metoprolol, propranolol, timolol), calcium channel blockers (dihydropyridines, diltiazem, verapamil), several hydroxymethyl glutaryl–coenzyme A (HMG-CoA) reductase inhibitors (fluvastatin, and in some studies, atorvastatin) and the benzodiazepine midazolam. Decreases in oxidative drug metabolism/clearance with age suggest that lower amounts of drug per unit time (or day) would be appropriate in older patients compared with younger patients, leading to the conventional recommendation to “start low and go slow” in the elderly. However, older age-related changes have not been detected in several population studies of patients including women and patients receiving multiple medications,14 suggesting that disease, environment, sex, and co-medications may have greater effects on CYP-mediated clearance than chronologic age. Data for humans on age-related changes in hepatic influx or efflux transporter enzymes are very limited. The lack of age-related changes in clearance of HMG-CoA reductase inhibitors (rosuvastatin, atorvastatin, fluvastatin) that are organic anion-transporting polypeptide (OATP) transporter substrates suggests age-related changes either are not marked or contribute less to overall substrate clearance than other factors. Therefore it is important to titrate to clinical effect after starting with reduced dosages in older patients. Attention has focused on genetic variation in explaining variability in drug metabolism, responses, and interactions (see Chapter 9). Warfarin and clopidogrel are examples of cardiovascular drugs metabolized by enzymes with relatively common pharmacogenetic variants. Polymorphisms of the CYP2C9 enzyme slow inactivation metabolism (clearance) of warfarin and polymorphic variants in the vitamin K–epoxide reductase complex (VKORC) can increase or decrease sensitivity to warfarin resulting in lower or higher dosing requirements (addressed later under Atrial Fibrillation: Anticoagulants) In general, the elimination half-life ( Age-related changes in protein binding of drugs are not usually found. Changes in free drug concentrations caused by competition of drugs for binding sites may occur but are transitory. Clinically significant examples involve warfarin, with changes in anticoagulation when additional drugs are added to warfarin therapy. Table 76-2 summarizes general guidelines for drug dosing in older patients. TABLE 76-2 Guidelines for Medication Prescribing in Older Patients Adverse drug events (ADEs) affect millions of people per year and account for up to 5% of hospital admissions. Adverse drug effects may manifest with “atypical” symptoms such as mental status changes and impaired cognition in the elderly. Literature reviews found ADE admission rates of 10.7% in elderly patients, with cardiovascular drugs accounting for approximately half, nonsteroidal anti-inflammatory drugs (NSAIDs) for 20%, and central nervous system drugs for 14%. A recent investigation of emergency hospitalizations for ADEs in older U.S. adults found that four medications were implicated, alone or in combination, in 67% of hospitalizations: warfarin (33.3%), insulins (13.9%), oral antiplatelet agents (13.3%), and oral hypoglycemic agents (10.7%).16 Medications previously recognized as “inappropriate” in the elderly17 were rarely implicated (1.2%). The risk for ADE-related emergency hospitalization increased with increasing age from 65 to older than 85 years of age. During non–ADE-related hospitalization, the odds ratio of severe ADEs with cardiovascular medications has been reported to be 2.4 times that of other medications. Both the risk of a medication error and that of a harmful or fatal outcome are highest in older adults.18 Other classes of drugs commonly associated with ADEs in community-dwelling elderly persons include diuretics, NSAIDs, selective serotonin reuptake inhibitors, beta blockers, and ACE inhibitors. In patients who are nursing home residents, drugs associated with ADEs are more frequently antibiotics, anticoagulants and antiplatelet drugs, atypical and typical antipsychotic drugs, antidepressants, antiseizure medications, or opioids. The most consistently identified risk factor for adverse drug interactions is the number of drugs prescribed, independent of age. Chronic administration of four drugs is associated with a risk of adverse effects of 50% to 60%; administration of eight or nine drugs increases the risk to almost 100% (Fig. 76-6). Although the goal is to prescribe as few drugs as possible in the elderly, the presence of multiple diseases and multi-drug regimens for common cardiovascular diseases often results in polypharmacy. The result is that approximately half of people older than 65 years of age have three or more medications prescribed on a daily basis, and 20% of patients 75 years and older have five drugs prescribed per outpatient encounter (www.cdc.gov). Even higher numbers of medications are prescribed for nursing home patients, averaging six to eight medications per day. Strategies that minimize the chance of drug interactions and adverse drug effects are thus essential. Pharmacokinetic interactions can lead to increased drug concentrations as a result of enzyme inhibition or to decreases secondary to enzyme induction. Interactions are more likely if co-administered medications are metabolized by or inhibit the same pathway (see Chapter 9 and also www.fda.gov/cder). The most potent inhibitors of the CYP oxidative enzymes are amiodarone (all isoforms) and droneradone (CYP3A), azole antifungal drugs itraconazole and ketoconazole (CYP3A), and protease inhibitors (CYP3A), followed by diltiazem (CYP3A) and erythromycin (CYP3A) (additional details are available online—e.g., at http://medicine.iupui.edu/clinpharm/ddis/main-table/). Coadministration of sulfonamide antibiotics with sulfonylureas can lead to hypoglycemia, in part because of CYP2C9 inhibition. Some drugs are administered as “prodrugs” and metabolized to active agents (many ACE inhibitors and clopidogrel). Inhibition of the antiplatelet effects of clopidogrel seen with coadministration of atorvastatin, decreasing clopidogrel activation by CYP3A, or proton pump inhibitors, which inhibit CYP2C19-mediated clopidogrel activation, has been reported. Induction of hepatic enzyme activity can lower concentrations of medications, leading to ineffective therapy. The antituberculosis drug rifampin is the most potent inducer of CYP1A and CYP3A. With mandatory screening for tuberculosis, treatment with rifampin may be initiated. Coadministered dosages of drugs cleared by CYP1A and CYP3A may need to be increased during rifampin administration and decreased upon discontinuation of rifampin. Markedly decreased cyclosporine levels and reduced clopidogrel inhibition of platelet aggregation during rifampin coadministration can occur. Other clinically relevant CYP inducers include carbamazepine (all CYPs), dexamethasone and phenytoin (CYP2C); caffeine, cigarette smoke, lansoprazole and omeprazole (CYP1A), St. John’s wort (CYP3A), and troglitazone (see http://medicine.iupui.edu/clinpharm/ddis/main-table/). Diet and drug and herb-drug interactions also occur. Because of the multiplicity of potential interactions and release of new medications and discovery of new interactions, use of pharmacy or computerized and online tools that provide comprehensive up-to-date information and guidelines for avoiding drug interactions is essential. Traditional text, online, and computer and smart phone applications are available from many sources—Lexicomp, the Medical Letter, the Physicians’ Desk Reference, Epocrates (available free of charge at www.epocrates.com), the FDA (the drug reactions section at www.fda.gov/cder), www.druginteractions.org, and http://medicine.iupui.edu/flockhart, among others. Many individual hospitals, health care systems, and pharmacies provide internal reference sources. Additional approaches that have been shown to reduce adverse drug reactions in older patients include involvement of pharmacy-trained personnel to assess the appropriateness of doses and medication counseling by members of a multidisciplinary care team. Algorithms have been developed for older patients to identify both medications that are potentially inappropriate and should be discontinued (STOPP criteria) and to identify ones that appear indicated and should be initiated (START criteria).19–21 Integrated medical record and pharmacy information, interactive databases, and computerized physician order entry with clinical decision support can reduce ADEs and improve medication therapy. By contrast, mandated drug utilization review efforts appear to be ineffective. Age-related changes in cardiovascular physiology and dynamics have a well-recogized impact on pharmacodynamics (see Table 76-1
Cardiovascular Disease in the Elderly
Demographics and Epidemiology
Pathophysiology
Medication Therapy: Modifications for the Older Patient
Loading Doses of Medications
Hepatic (and Intestinal) Clearance
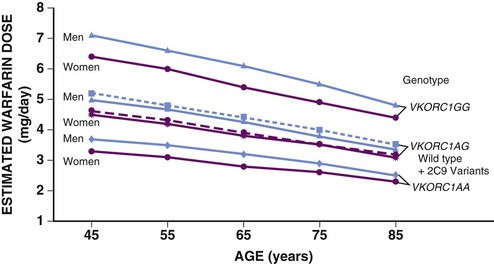
![]() (Fig e76-1). Investigations of the use of genotyping to improve warfarin therapy to date have failed to identify frail elderly persons requiring the lowest warfarin dosing and cannot replace use of reduced initial doses and close monitoring in the very old.15 Clopidogrel is administered as a prodrug that requires activation metabolism by the polymorphic CYP2C19, and to a lesser extent CYP3A, for antiplatelet effects (see Chapter 82). Low activity pharmacogenetic variants result in less antiplatelet effect for any dose in carefully controlled investigations but the impact in the clinical setting is less striking leading to an uncertain role for pharmacogenetic screening to guide cardiovascular drug dosing at this time.
(Fig e76-1). Investigations of the use of genotyping to improve warfarin therapy to date have failed to identify frail elderly persons requiring the lowest warfarin dosing and cannot replace use of reduced initial doses and close monitoring in the very old.15 Clopidogrel is administered as a prodrug that requires activation metabolism by the polymorphic CYP2C19, and to a lesser extent CYP3A, for antiplatelet effects (see Chapter 82). Low activity pharmacogenetic variants result in less antiplatelet effect for any dose in carefully controlled investigations but the impact in the clinical setting is less striking leading to an uncertain role for pharmacogenetic screening to guide cardiovascular drug dosing at this time.
Elimination Half-Life
 ) for a drug increases with age, so the time between dosage adjustments needs to be increased in older patients before the full effect of a given dose can be assessed. Conversely, increased time is needed for complete drug elimination from the body and dissipation of drug effects.
) for a drug increases with age, so the time between dosage adjustments needs to be increased in older patients before the full effect of a given dose can be assessed. Conversely, increased time is needed for complete drug elimination from the body and dissipation of drug effects.

Adverse Drug Events and Drug Interactions
Pharmacokinetic Interactions
Adverse Pharmacodynamic Effects
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Cardiovascular Disease in the Elderly
76
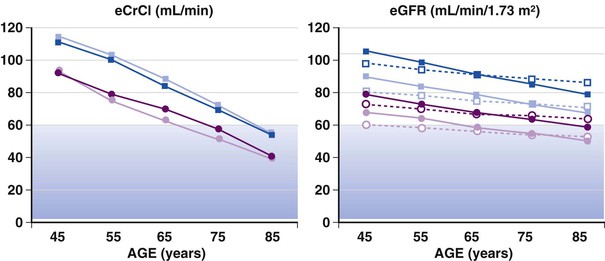
FIGURE 76-5 Estimates of creatinine clearance (eCrCl) using the C&G formula (left panel) and estimates of glomerular filtration rate (eGFR) using the MDRD simplified algorithm (represented by open symbols and dotted lines) and the CKD-EPI algorithm (represented by solid symbols and lines) for men and women 45 to 85 years of age (right panel). For calculations, mean weight and height by decade were obtained from U.S. survey data, serum creatinine was entered as 1.0 mg/dL (average for age older than 65 years from the survey data). Circles represent estimates for women; squares are estimates for men; lighter symbols are estimates for whites; and darker symbols represent estimates for African Americans. The shaded areas indicate GFR estimates of 30 to 59 mL/min/m2, classified as stage 3 renal disease or moderate GFR decrease. Cockcroft and Gault estimates show a steeper decline with age. Both formulas estimate lower clearance in women than in men and higher clearances in African Americans than in whites (based on average height and weights and the same creatinine). Data source: National Health and Nutrition Examination Survey (NHANES) (available at http://www.cdc.gov). (Modified from Schwartz JB: The current state of knowledge on age, sex, and their interactions on clinical pharmacology. Clin Pharmacol Ther 82:87, 2007.)
FIGURE e76-1 Warfarin dosing. Estimated warfarin dose by age, sex, and genotype. Estimates of doses are lower in women than in men of the same age and genotype, and age is predicted to affect dosage requirements for all genotypes. Variants of the VKORC can result in both increased sensitivity (1AA) and decreased sensitivity (1AG). Dotted lines represent data for both wild type CYP2C9 and its variants. Estimates were generated from an online calculator available at www.warfarindosing.org. (Modified from Schwartz JB: The current state of knowledge on age, sex, and their interactions on clinical pharmacology. Clin Pharmacol Ther 82:87, 2007.)
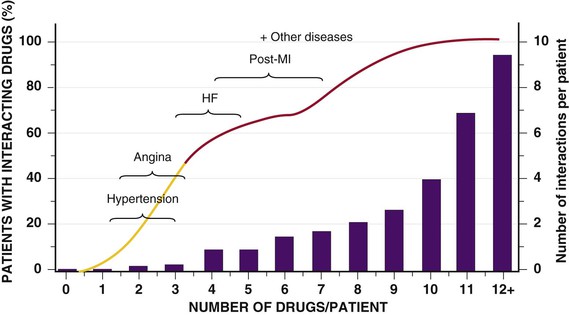
FIGURE 76-6 The relationship between the number of drugs consumed and drug interactions. Current guidelines for the pharmacologic management of patients with HF or recent MI place them at high risk for drug interactions. (From Schwartz JB: Clinical Pharmacology. Adult Clinical Cardiology Self-Assessment Program (ACCSAP) V, 2003. American College of Cardiology Foundation. Available at: http://www.cardiosource.org/. As modified from Nolan L, O’Malley K: The need for a more rational approach to drug prescribing for elderly people in nursing homes. Age Aging 18:52, 1989; and Denham MJ: Adverse drug reactions. Br Med Bull 46:53, 1990.)