Fig. 5.1
A Swan-Ganz pulmonary artery catheter is introduced into the right internal jugular vein. A thin-walled venous cannula (15–17 Fr Biomedicus ™) is passed into the distal superior vena cava using the internal jugular “double-stick method”
Operative Conduct
Cardiopulmonary Perfusion
Typically, peripheral cardiopulmonary perfusion is established via the right internal jugular vein and right femoral vessels. Most patients require “bicaval” cannulation for adequate venous drainage. Both the right femoral artery and vein are exposed through a 2-cm oblique groin incision. Adventitial layers are kept intact with proximal and distal control of the vessels typically not required. Adventitial purse-string sutures (4-0 Prolene, Johnson & Johnson, Piscataway, NJ) are placed near the inguinal ligament. After adequate heparinization, arterial (17–19 Fr) and venous (21 Fr) Bio-Medicus cannulas (Medtronic, Minneapolis, MN) are positioned using the Seldinger guide-wire technique under TEE guidance (Fig. 5.2). In obese patients, it is advantageous to create a counter incision below the incision and tunnel the cannulas through the subcutaneous tissue of the upper thigh. This is done to allow each device to enter each vessel at a 45° angle, which makes easier and safer passage of coaxial dilators and cannulas. If the angle is too acute, entry is difficult and the potential for posterior wall disruption or dissection is increased. After confirmation of appropriate cannula positioning, cardiopulmonary perfusion can be instituted.
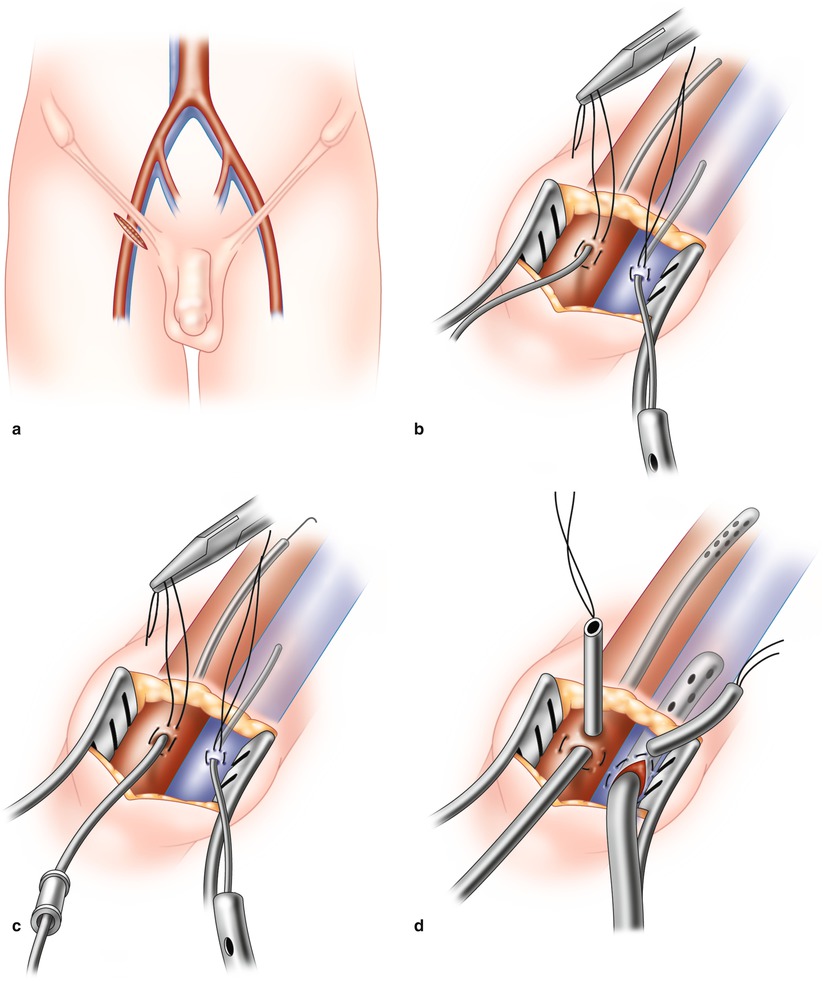

Fig. 5.2
(a) A 2-cm transverse incision is made in the right groin and both femoral vessels exposed. (b) An oval purse string is placed in the adventitia of the artery and vein. In both vessels a guide-wire is passed cephlad and position confirmed by echocardiography. (c) Progressive coaxial dilators are passed over individual guide-wires. (d) Both arterial and venous cannulas are passed over the final dilator to reside in the iliac artery and right atrium, respectively. Again echo guidance is essential
Alternate Arterial Cannulation Techniques
Patients with significant systemic arterial disease should have a pre-operative CT scan to define femoral arterial lumen size, presence of ileo-femoral stenosis, and complicating aortic disease. Occasionally, atherosclerotic disease within the ileo-femoral, descending thoracic, or abdominal aorta, preclude retrograde femoral artery perfusion. In these cases we have used two arterial cannulation methods:
Direct Ascending Aorta Cannulation
After the thoracic cavity has been entered, the ascending aorta can be cannulated directly through a purse string suture. We use a 23-Fr StraightShot™ aortic cannula (Edwards Lifesciences, Irvine, CA) passed through the chest wall using a 10-mm trocar. This cannula has a spring-loaded blade in the dilator tip (Fig. 5.3). It is most important to place two concentric pledgeted purse strings in the ascending aorta. Alternately, if a Biomedicus™ arterial cannula is used, the guide-wire should be confirmed to be in the descending aorta before introducing dilators or the perfusion cannula.
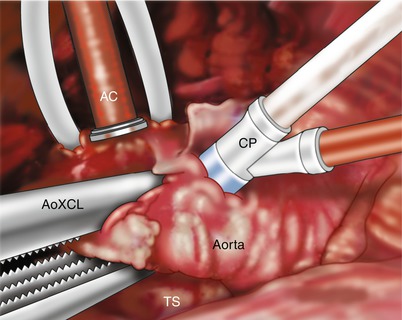

Fig. 5.3
A Straightshot ™ aortic cannula (AC) has been passed through chest wall in the anterior 2d interspace and secured with snares. An aortic root cardioplegia cannula (CP) is positioned in the proximal aorta. The aorta is occluded using a transthoracic cross clamp (AoXCL) with transverse sinus (TS) access
Axillary Arterial Cannulation
If it is not feasible to cannulate the ascending aorta directly, we use the right axillary artery for antegrade perfusion. The artery is exposed through an infra-clavicular incision. Generally we sew a 8-mm GelSoft™ knitted graft (Vascutek, Terumo, Ann Arbor, MI) end-to-side to the axillary artery with 5-0 polypropylene suture (Fig. 5.4). Thereafter, using either an appropriate size arterial cannula or a 3/8th inch pump tubing connector, continuity with the bypass circuit is established. Alternately, the axillary artery can be cannulated directly; however distal arm perfusion must be monitored while the cannula is in place.
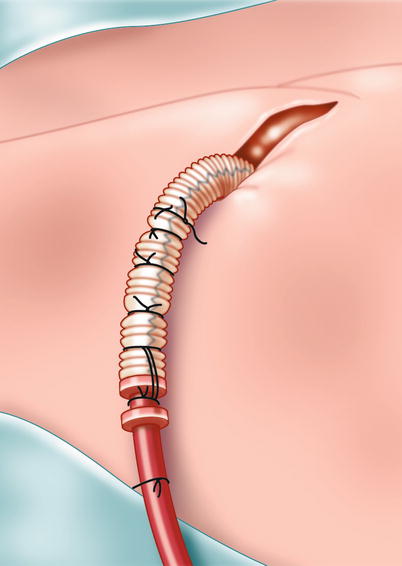

Fig. 5.4
Axillary artery cut down. Right axillary arterial cannulation: after exposure of the axillary artery, an 8-mm woven graft is sutured end-to-side, and a similar size inflow cannula is “plugged” into the graft and secured with multiple heavy ligatures
Femoral Arterial Cannulation and the Endoclamp™
When using the Endoclamp™ aortic balloon occlusion technique, a specialized EndoReturn™ (Edwards Lifesciences, Inc., Irvine, CA) femoral arterial cannula (21 Fr or 23 Fr) must be used (Fig. 5.5). It has a one-way valve for balloon catheter passage into the aorta. This arterial cannula is significantly larger than that used in the trans-thoracic clamp method (17–19 Fr). Therefore, distal leg perfusion must be monitored continuously using oxygen saturation patches. Should either limb perfusion become compromised or the coaxial balloon catheter limit optimal arterial inflow then the opposite femoral artery should be cannulated for perfusion or balloon insertion. In this circumstance the Endoballoon should be placed directly into the opposite femoral artery.
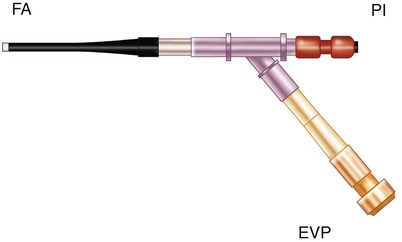

Fig. 5.5
Arterial inflow cannula for passing the Endoballoon ™ aortic clamp through the side arm valve. PI arterial inflow from pump, FA femoral artery, EVP Endoballoon ™ valve port
Aortic Occlusion Methods
An ascending aorta cardioplegia needle/vent is secured just lateral to the right coronary origin. Most often we use a specialized trans-thoracic aortic cross clamp (Scanlan International, Minneapolis, MN) to occlude the ascending aorta (Fig. 5.6). This clamp is passed into the right chest, through a 4-mm incision placed in the 2nd intercostal space in the superior posterior axillary line. The clamp arm should cross the superior vena cava at the level of the pericardial junction. This posterior and superior clamp position is important to avoid collisions with the left robotic instrument arm. Under endoscopic control and via the transverse sinus, clamp tines are positioned carefully on each side of the aorta (Fig. 5.7). During insertion and clamping, care must be taken to avoid injury to the right pulmonary artery, the left atrial appendage, or the left main coronary artery.




