Cardiopulmonary Bypass and Myocardial Protection for Minimally Invasive Cardiac Surgery
Alan P. Kypson
Derek A. Sanderson Jr.
L. Wiley Nifong
W. Randolph Chitwood Jr.
HISTORICAL BACKGROUND
Eversince Gibbon used the first heart-lung machine (1953) and Lillehei cross-circulated patients with parents (1954), cardiopulmonary perfusion and vascular cannulation methods have evolved in a serpiginous pathway (1,2). Disc and screen oxygenators were replaced eventually by bubble devices. In the early 1970s, we cannulated the common femoral artery for perfusion inflow and for venous return to the pump both vena cavae were drained by gravity. Thus, by adjusting the operating table height above the pump, return to the cardiotomy reservoir and bubble oxygenator was regulated. We primed the pump with a mixture of crystalloid and a great deal of whole blood. Often family members were solicited to provide fresh blood donations. As the cannula occluded the distal common femoral artery, blood flow to the leg was negligible and only through collaterals. It is interesting that we did not observe more irreversible leg ischemia.
At Duke University, Drs. Sealy, Young, and Brown developed and used the first commercial heat exchanger, where cooling and rewarming were regulated by a mixing valve and “tap water” temperatures (3). Myocardial protection was by aortic clamping with coronary ostial blood perfusion, ventricular fibrillation, or intermittent ischemic arrest. Cardiac dysfunction was common even after some of the simplest valve and coronary operations. The use of high-dose postoperative inotropic support was the norm for many patients. Although Melrose had used high-dose potassium cardioplegia before in England, the use of depolarizing solutions was not accepted until Gay and Ebert published their seminal 1975 paper (4,5). In Germany Kirsch, Bretschneider, and Bleese and in Britain Braimbridge and Hearse developed various cardioplegic solutions (6,7,8,9,10). Thereafter, Buckberg and associates introduced blood-based cardioplegia (11). By the mid-1970s, cold crystalloid cardioplegia was advocated by most surgeons. During this era, retrograde peripheral arterial perfusion had been replaced by central aortic cannulation. By the mid-1980s, most programs were using membrane oxygenators, high-flow cannulas, better in-line filters, less pump prime, and improved myocardial preservation techniques. Retrograde catheters were developed by the 1980s, and transvenous cardioplegia was particularly helpful protection in the presence of diffuse coronary artery disease (12,13). Little had changed in perfusion technology until the mid-1990s with the exception of widespread use of cold-blood cardioplegia.
In 1995, several surgeons began MICS in the United States. Cosgrove and Cohn started to use less invasive sternal incisions with modified cannulation techniques (14,15). Carpentier performed the first videoscopic mitral valve repair at Broussais Hospital in 1996 (16). Several months later, our group performed the first videoscopic minimally invasive mitral valve replacement (17). We cannulated the right atrium directly to provide gravity pump by inserting the venous cannula through the 5-cm minithoracotomy. Retrograde arterial inflow was provided through the right common femoral artery. We occluded the aorta with a newly developed transthoracic clamp and used antegrade crystalloid cardioplegia to protect the heart. Our result was pleasing, and we presented the first videoscopic MICS mitral valve series at the AATS meeting in 1997 (18). Soon thereafter, we converted to vortex pump kinetic venous drainage through higher-flow long femoral vein cannulas. As some surgeons had done earlier, we purged air from the thoracic cavity with CO2.
These modified approaches heralded the desire for better cannulas and perfusion circuits. Originally introduced in 1996, the Heart-Port (Edwards Lifesciences, Irvine, CA) system permitted a new minimally invasive surgical platform with avoidance of a median sternotomy as well as closed-chest cardiopulmonary bypass with an arrested heart (19). The technique had a meteoric launching and appeared to be the solution for surgeons and patients desiring MICS. However, early complications and cost blunted the enthusiasm of many surgeons who were initial advocates.
Over the next 15 years, MICS was done through small incisions with direct vision and was used mostly for valvular disease. Using peripheral perfusion techniques, developed for MICS earlier, robotic mitral repairs were begun in the United States in 2000 (20). Newer cannulas enabled safe peripheral vascular insertion with high-flow characteristics. The use of assisted venous return (kinetic or suction) was a major advance that enabled the best flow characteristics through thinwalled cannulas. Either vortex centrifugal pumps or vacuum assistance became used widely in all MICS cases. Only after
making these modifications were the possibilities of MICS fully realized. MICS advanced remarkably after several clinical series showed that the approach rendered the same quality and safety as the traditional sternal approach, while providing advantages to the patient (21,22,23,24). The success that MICS has achieved would have been impossible without major advances in perfusion technology and techniques.
making these modifications were the possibilities of MICS fully realized. MICS advanced remarkably after several clinical series showed that the approach rendered the same quality and safety as the traditional sternal approach, while providing advantages to the patient (21,22,23,24). The success that MICS has achieved would have been impossible without major advances in perfusion technology and techniques.
CARDIOPULMONARY BYPASS MONITORING
In all of our MICS operations, we monitor closely cardiac dynamics and function as well as leg and brain perfusion. In each patient, a left arm radial arterial catheter is inserted for systemic pressure monitoring. If intra-aortic balloon occlusion (clamping) is planned, arterial pressures should be monitored in both arms. This is done to ensure that the balloon has not inadvertently occluded the innominate artery. For hemodynamic monitoring, a thermo-dilution Swan-Ganz pulmonary artery catheter (Edwards Lifesciences) is inserted and passed via the right internal jugular vein. In each patient, a transesophageal echocardiographic probe is placed to evaluate abnormal valve pathology and ventricular function. Moreover, all cannulas are positioned under echocardiographic guidance. We use BIS (Bispectral Index) (Covidien, Inc., Boulder, CO) flow to monitor cerebral blood flow and anesthetic levels. To determine the adequacy of blood flow in a cannulated extremity, we place oxygen saturation patches (Somanetics, Inc., Troy, MI) on each lower leg for comparison. Should the saturation fall significantly in the perfused leg, we insert a distal arterial shunt (5F catheter), which then is hooked to the arterial perfusion circuit. Generally, oxygen saturation levels return to baseline in the perfused leg after shunt insertion and blood flow improvement.
Cannulation
Hemisternotomy
When using a ministernotomy either for aortic or mitral valve surgery, direct aortic and right atrial cannulation is effective. For minimally invasive aortic surgery, we insert a 17 or 19F Bio-Medicus high-flow cannula (Medtronic, Inc., St Paul, MN) into the aorta at the level of the innominate artery. This can be done either by direct insertion or over a guide wire. For venous return, either a direct right atrial “pancake” two-stage VC2 caval cannula (Medtronic, Inc.) or a long percutaneous right femoral to right atrial cannula can be used. The “pancake” cannula is flattened against the incision wall for greater exposure through a small incision.
Minithoracotomy
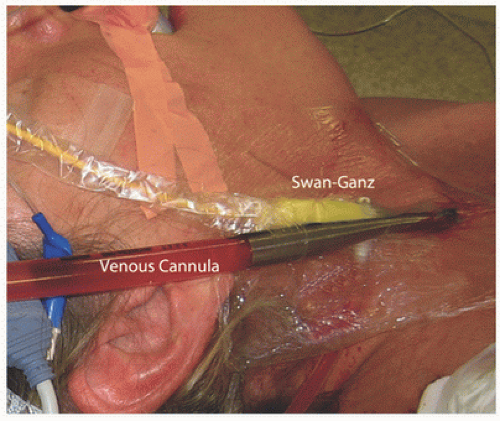
Typically, peripheral cardiopulmonary perfusion is established via the right internal jugular vein and femoral vessels. Although some surgeons prefer a single long femoral venous cannula, we prefer “bicaval” return to optimize drainage and myocardial cooling. Under sterile conditions, and using the Seldinger guide-wire technique, the anesthesiologist directs a 17F thin-walled Bio-Medicus cannula into the distal superior vena cava via the right internal jugular vein under transesophageal echocardiogram (TEE) guidance. A Swan-Ganz pulmonary artery catheter is placed via either the subclavian or the internal jugular vein (using a “double-puncture” method) (Fig. 7.1). Both the right femoral artery and vein are exposed through a 2-cm oblique groin incision (Fig. 7.2). Proximal and distal vascular control is not required typically. Adventitial 4-0 Prolene oval (longitudinal)
purse-string sutures (Johnson & Johnson, Piscataway, NJ) are placed in both vessels and near the inguinal ligament. After adequate heparinization, arterial (17-19F) and venous (21F) Bio-Medicus cannulas are passed into the proximal common femoral artery and right atrium, respectively, by the Seldinger guide-wire technique using TEE guidance (Fig. 7.3). In corpulent patients, it is advantageous to create a counterincision and tunnel the cannulas through the subcutaneous tissue of the upper thigh. This is done to allow entrance into each vessel at a 30° to 45° angle, which makes easier and safer passage of coaxial dilators and cannulas. If the angle is too acute, entry is difficult, and the potential for posterior arterial and/or vein wall disruption or dissection is increased.
After confirmation of appropriate cannula positioning, cardiopulmonary perfusion can be initiated.
purse-string sutures (Johnson & Johnson, Piscataway, NJ) are placed in both vessels and near the inguinal ligament. After adequate heparinization, arterial (17-19F) and venous (21F) Bio-Medicus cannulas are passed into the proximal common femoral artery and right atrium, respectively, by the Seldinger guide-wire technique using TEE guidance (Fig. 7.3). In corpulent patients, it is advantageous to create a counterincision and tunnel the cannulas through the subcutaneous tissue of the upper thigh. This is done to allow entrance into each vessel at a 30° to 45° angle, which makes easier and safer passage of coaxial dilators and cannulas. If the angle is too acute, entry is difficult, and the potential for posterior arterial and/or vein wall disruption or dissection is increased.
After confirmation of appropriate cannula positioning, cardiopulmonary perfusion can be initiated.
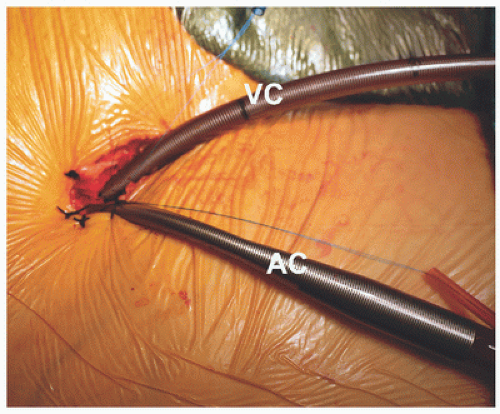 FIGURE 7.2. Arterial and inferior vena cava cannulation: femoral arterial (AC) and vein (VC) cannulas are shown in place through a small incision. |
Alternate Arterial Cannulation Techniques
Atherosclerotic disease within the ileo-femoral vessels, or descending thoracic and abdominal aorta, may preclude safe retrograde femoral artery perfusion.
Direct Ascending Aorta Cannulation through the Minithoracotomy
The ascending aorta can be cannulated directly through a purse-string suture. We use either a Bio-Medicus guide-wiredirected cannula or a 23F Straight Shot device (Edwards Lifesciences), which is passed through the chest wall via a 10-mm trocar. It is most important to place two concentric pledgeted purse strings in the ascending aorta near the innominate artery origin.
Axillary Arterial Cannulation
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree