17 Cardiogenic Shock after Myocardial Infarction
Pathogenesis
Predominant Left Ventricular Failure
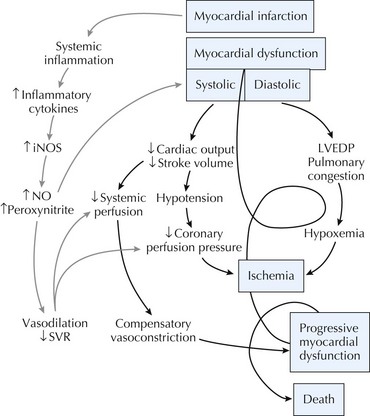
CS is traditionally defined as an unsupported systolic blood pressure less than 90 mm Hg with normal to elevated LV filling pressures and evidence of end-organ hypoperfusion. Acute ischemia due to plaque rupture/thrombosis can result in acute myocardial dysfunction. Decreased cardiac output on the basis of inadequate LV stroke volume in the setting of MI can lead first to decreased systemic systolic blood pressure. Hypotension can then lead to further reduction in coronary perfusion pressure and further worsening of myocardial ischemia. There may also be cardiac ischemia due to fixed flow-limiting stenoses in epicardial coronary arteries remote from the infarct-related vessel. Ischemia thus begets ischemia resulting in a progressive spiral of hemodynamic collapse culminating with death. In this traditional paradigm of CS, vasoconstriction from falling cardiac output was thought to be the major mechanism by which the neurohormonal system compensates for hypotension. The recognition that many patients have unexpected vasodilation and low systemic vascular resistance in this setting has led to modification of this conceptual design. Observational evidence suggests that inflammatory cytokines such as interleukin-6 (IL-6), IL-1, and tumor necrosis factor-α are elevated in patients with CS to the same levels seen in patients with a septic state. These findings suggest that MI may result in a systemic inflammatory response syndrome as previously observed with infection or trauma that results in myocardial depression and hypotension independent of ischemic necrosis (Fig. 17-1). These findings are also of importance when considering the diagnostic evaluation of CS patients and optimal therapy (see below).
Right Ventricular Failure
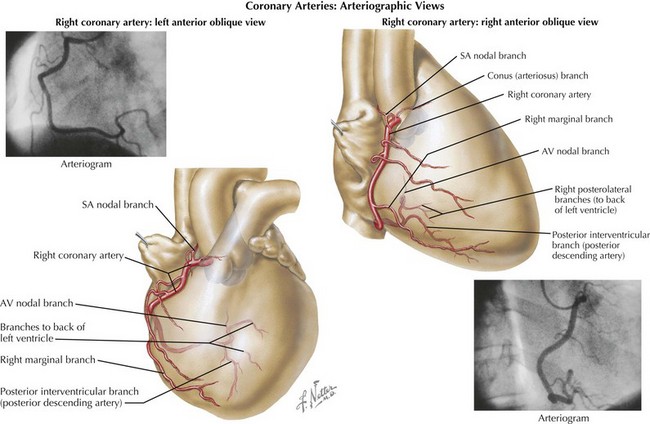
RV dysfunction commonly occurs when there is infarction of the territory supplied by the acute marginal branches of the right coronary artery (Fig. 17-2). This typically results in hypotension with clear lung fields and is often accompanied by bradyarrhythmic complications, including high-grade atrioventricular block and even complete heart block. ST-segment elevation in right-sided ECG leads V3 and V4 is a very specific finding for RV infarction. A right-sided ECG should be obtained in all patients presenting with an acute inferior MI and in any patients suspected of having RV infarction. With RV infarction, the right-sided filling pressures become acutely elevated, since there is reduced forward flow through the pulmonary circulation into the left side of the heart. Elevation in RV end-diastolic pressure may also negatively impact LV filling by causing a “bowing” of the interventricular septum into the LV cavity. As a result, the left ventricle is underfilled and cardiac output further reduced. Reperfusion of the right coronary artery improves RV function, restores conduction, and can often result in normalization of hemodynamics.
Mitral Regurgitation
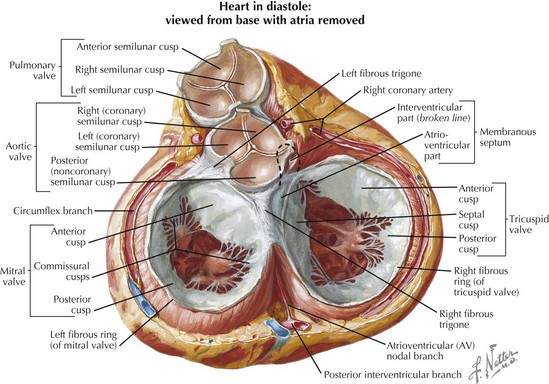
The anatomy of the mitral valve as depicted in Figure 17-3 reveals how mitral leaflet closure depends on papillary muscle function. Each mitral valve leaflet is connected by chordae tendineae to both the posteromedial and anterolateral papillary muscles. The posteromedial papillary muscle is at greater risk from ischemic damage, since it has a single blood supply from the posterior descending artery, whereas the anterolateral papillary muscle usually receives dual blood supply from the left anterior descending and circumflex arteries. Consequently, inferior and posterior MIs are more likely to cause papillary muscle dysfunction/rupture and resultant severe mitral regurgitation. Additional risk factors for papillary muscle rupture include age, female sex, first MI, hypertension, and single-vessel disease. The jet of mitral regurgitation in this situation is eccentric and directed away from the affected flail mitral leaflet. In contrast, ischemic mitral regurgitation results from a restricted posterior mitral leaflet with resultant central to posteriorly directed mitral regurgitation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree