). The plane of the valve is not flat, but resembles a saddle. During the cardiac cycle, the mitral valve size and shape change. In particular, the size of the annulus and positions of the high points of the saddle vary considerably during the cardiac cycle. In normal individuals, the area of the mitral valve lowers (10–15 %) during systole. The atrial wall can contribute to mitral regurgitation when the left atrial enlarges.
The structures of the mitral valve encompass the annulus (i.e., annular attachment at the atrioventricular junction), leaflets, chordae tendinae, and posteromedial and anterolateral papillary muscles in the left ventricle.
The oval (D-shaped) flexible fibrous mitral valve annulus (hingeline of the valvular leaflets) enables the orifice to undergo complex shape changes during the cardiac cycle. It determines the opening area of the mitral valve. It is composed anteriorly of a fibrous component between the two fibrous trigones, the trigonum fibrosum dextrum and sinistrum, which separate the mitral valve from the aortic valve [481]. The right fibrous trigone and the membranous septum form the central fibrous body. The annulus edge opposite to the fibrous body can be altered by dilation and be the site of calcifications. In the anterior concave part of the annulus, fibers have a parallel and circular orientation. Its lateral and posterior parts are linked to the anterior part by the left and right fibrous trigone. The lateral part of the mitral annulus consists of collagen fibers. In the annulus transition zone, where the leaflets are anchored to the myocardium, the elastin and collagen fibers radiate into the myocardium [481]. In the hinge zone, where the atrial endocardium is thickened, the number of elastin fibers increases.
The anterior leaflet is commonly semicircular and the posterior leaflet has a crescentic shape. Both leaflets merge at the posteromedial and anterolateral commissures (Table 6.1). The tips of the papillary muscles point toward the commissures. A commissure corresponds to long invaginations of the free margin that limit adjacent cusps, the depth of which is larger than two thirds of the maximal width of the larger of the two adjacent leaflets. An indentation that separates two valvular cusp segments have a depth ranging from one third to two thirds of the maximal width of the larger of the two adjacent leaflets. Their number and position determine the number and size of valvular segments.
Table 6.1
Distance from the free margin to the annulus in the central part of commissural regions and extent of the insertion zone of commissural cords along the commissural free margin (mm; Source: [485])
Geometrical parameter | Women | Men |
|---|---|---|
Anterolateral commissural region | ||
Free margin–annulus length | 5–10 | 5–13 |
Insertion length of commissural cords | 3–15 | 6–19 |
Posteromedial commissural region | ||
Free margin–annulus length | 4–11 | 6–12 |
Insertion length of commissural cords | 9–22 | 12–26 |
The anterior leaflet (a.k.a. aortic and septal leaflet) is the bigger cusp (Table 6.2). It is close to the aortic root. The aortic leaflet is divided arbitrarily into three regions (A1–A3) from the cardiac base to the apex. The anterior leaflet is close to the aortic valve (left and noncoronary cusps). It is much smoother than the mural cusp, usually having no indentations along its free edge. The atrial myocardium contacts the hinge of the aortic leaflet [486].
Table 6.2
Size of the anterior mitral valve cusp and of segments of the posterior mitral valve leaflet. (Source: [485])
Leaflet | Women | Men |
|---|---|---|
Septal (anterior) leaflet | ||
Height | 18–35 | 20–30 |
Width | 18–42 | 25–48 |
Mural (posterior) leaflet | ||
P1 segment | ||
Height | 8–14 | 9–20 |
Width | 9–20 | 9–40 |
P2 segment | ||
Height | 7–18 | 9–20 |
Width | 6–26 | 13–38 |
P3 segment | ||
Height | 5–11 | 6–17 |
Width | 5–22 | 9–31 |
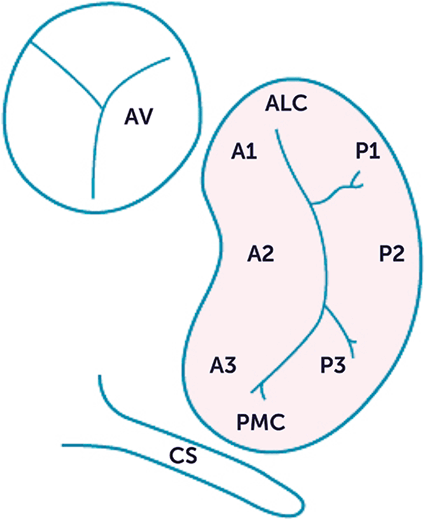
The narrow posterior leaflet (a.k.a. mural leaflet) is in 91 % of cases divided into three parts (posteromedial or right, intermediate, which is wider and higher than the others, and anterolateral or left) [481]. In adults, the mural leaflet has indentations that do not extend through the leaflet to the annulus. They generally form three segments1 (scallops due to scalloped shape) along the elongated free edge according to Carpentier’s nomenclature (Fig. 6.1 and Table 6.3; [487]):
Each Pi segment faces Ai segment of the anterior leaflet. The posterior smaller leaflet is attached to nearly two thirds of the circumference around the left atrioventricular junction. The degree of myocardial extension toward the insertion of the hinge (or fulcrum) of the mural posterior leaflet varies between hearts and in a given heart, between regions [486].
1.
P1, the lateral segment adjacent to the anterolateral commissure
2.
P2, the central segment that can significantly vary in size, and
3.
P3, the medial segment adjacent to the posteromedial commissure

Fig. 6.1
(Left) Segments of the mitral valve (Carpentier’s classification; Source: [488]; A anterior (aortic or septal) leaflet, P posterior (mural) leaflet, 1 lateral segment, 2 middle segment, 3 medial segment, AV aortic valve, CS coronary sinus). The line of coaptation is slightly U-shaped. The tips of the leaflets curl toward the ventricle. (Right) The saddle-shaped mitral valve annulus deforms during the cardiac cycle with position changes of high (A anterior, P posterior) and low points (L lateral, M medial) of the saddle
Table 6.3
Some end-systolic dimensions of the mitral valve. (Source: [489])
Between-commissure width | 17.2 ± 5.8 mm |
|---|---|
Width of the middle P2 segment | 10.9 ± 2.5 mm |
Distance between the mitral annulus saddle | 7.3 ± 2.2 mm |
high point and the mitral orifice plane | |
Distance between the papillary muscle tip | 20.0 ± 5.8 mm |
and the free margin indentation end | |
Distance between papillary muscle tips | 11.6 ± 5.0 mm |
The leaflets can be subdivided into a basal region, the zone of attachment to the annulus, and thin central translucent (clear) and thick rough zone from the attachment point of each leaflet at the annulus to the free edge. The chordae tendinae of the mitral valve are tethered to the ventricular face of the leaflet rough zone and free margin [481]. The rough zone is also the region of apposition (symmetrical overlap of the leaflet free edge, usually a minimum of 4–5 mm) and coaptation (region of between-leaflet contact when the mitral orifice is closed). Coaptation and correct apposition of leaflets prevent regurgitation.
Measurements of the leaflet geometry encompass:
The two leaflets of the mitral valve differ in structure. The mitral leaflets are composed of a fibrous skeleton. The mitral valve is made up of four layers: ventricularis, fibrosa, spongiosa, and atrialis [487]. Two main layers, a lamina spongiosa and a lamina fibrosa, face the atrial and ventricular side, respectively. They are covered by the endocardium. The latter is continuous with that of the atrium and ventricle. The thickness of each layer varies from the cusp insertion at the annulus to the free edge.
The circumference of the annulus
The length of annular attachment of each leaflet
The width of the coapted (folded) margin
The dimensions and surface area of each leaflet (the surface area of the anterior leaflet being about 1.6 times larger than that of the posterior leaflet [490]); among others.
The atrialis is composed of mainly aligned elastic and collagen fibers. The spongiosa, a major component of the free edge, consists principally of proteoglycans and elastic fibers. The fibrosa, a major load-bearing layer, comprises a collagenous core. The collagen fibers are aligned and provide strength and stiffness to the leaflet. The fibrosa extends from the annulus into two thirds of the leaflet; it is absent at the free edge. The ventricularis covered by an endothelium consists of elastic and collagen fibers. Near the annulus, the fibrosa is the thickest layer; close to the free edge, it becomes thinner. On the other hand, the thickness of the spongiosa and atrialis rises distally to become the main component of the leaflet at the free edge [487].
The subendocardial connective tissue of the lamina spongiosa consists of fibroblasts, fibrocytes, histiocytes, and collagen fibers (caliber 15–35 nm) [481]. Fibroblasts, smooth myocytes, and cardiomyocytes in the proximal third reside in mitral valve leaflets. Cardiomyocytes extend a short distance into the base of the mitral leaflets without continuity with atrial and ventricular walls. At the atrioventricular junction, fibrofatty tissues indeed interpose.
The architecture of the fibrous meshwork, that is, the local fiber orientation, influences the valve rheology. Collagen in the leaflet comprises primarily type-1 (74 %), type-3 (24 %), and type-4 (2 %) [487].
Extra leaflets can be observed [490]. About two thirds of heart specimens exhibit the presence of an extra commissure or indentation, hence of additional leaflets or segments, respectively, at least in a small Indian population.
Mitral valve leaflets possess innervations from both sensory and autonomic components [481]. In the anterior leaflet, nerve density is twofold greater than that in the posterior leaflet. Nerves are situated in the atrial layer and extend over the proximal and medial portions of the leaflet.
Both leaflets are attached to the papillary muscles by the chordae tendinae. The posteromedial papillary muscle gives cords to the medial half of both leaflets (i.e., posteromedial commissure, A3, P3, and posterior half of A2 and P2 segments). The anterolateral papillary muscle sends cords to the lateral half of the leaflets (i.e., anterolateral commissure, A1, P1, and anterior half of A2 and P2 segments). Their position in the left ventricle varies among patients. In some subjects, one or both papillary muscles cannot be clearly defined; they are replaced by multiple small muscle bundles connected to the ventricular wall [487].
Thin chordae have a lower average fibril bore and a greater average fibril density than thick chordae. The surface of the chordae consists of a layer of endotheliocytes and a dense layer of elastic fibers [481]. These tendinous chordae are composed of elastic and collagen fibers parallel to the axis. They transmit the tension of the contracting papillary muscles to the valve leaflets. The majority of the chordae insert at the free margin or behind the free margin at the ventricular side (rough zone) of the leaflets. The base chordae insert at the leaflets near their attachment at the annulus originating from ventricular myocardium. The commissural chordae (interleaflet or commissural cords) insert at the free margin of two adjoining leaflets. Normally, only two commissural cords exist supporting each free margin of the commissural region. These cords arise from a single stem that branches, thereby allowing adjacent leaflets to coapt and to move apart.
In fact, numerous classifications of tendinous cords exist. Three types of chordae tendinae are also described [487]:
Among the secondary cords of the aortic (anterior) leaflet, two are the largest and thickest (strut cords). The latter originate from the tip of each papillary muscle. Hence, according to function, tendinous cords are categorized into commissural, indentation, and strut cords.
1.
Primary cords attached to the free edge of the rough zone of both leaflets
2.
Secondary cords connected to the ventricular surface of the rough zone
3.
Tertiary cords tethered to the basal zone of the mural (posterior) leaflet only
Mitral valve repair is more popular than mitral valve replacement. However, changes in cusp geometry alter valvular mechanics and outcomes, especially valve repair durability.
Annular flattening can result from myocardial infarction. On the other hand, the three-dimensional saddle shape of the mitral annulus changes during the cardiac cycle with systolic high points along the anterioposterior plane and low points at commissures. This shape enables a better leaflet curvature during systolic closure. The saddle shape influences the mechanics of the mitral leaflet. It lowers stresses on the chordae tendineae and strains on the leaflets, in particular in the posterior leaflet during systolic valve closure [492]. Maximal strain decreases from planar shape to 20 % saddle annulus.2
Estimation of regional stresses and strains enables to adapt the therapeutic strategy for valvular repair and replacement. In vivo deformations of the mitral valve anterior leaflet during the cardiac cycle was evaluated using sonocrystal transducers sutured to the valve [493]. Changes in annular geometry alter valvular strains in vivo.
The deformation of the insertion zone of chordae to porcine mitral valves endowed with a structured array of markers was measured during the cardiac cycle by tracking marker motions using high-speed cameras [495]. The insertion zone mechanics depends on the collagen architecture. The insertion region is stretched during the entire cardiac cycle.
Kinematics of the mitral valve annulus was explored in sheep using implanted sonocrystals [494]. Kinematic data collected can be fitted to a 3-D spline based on 5-order hermite shape functions with C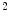 continuity. Axial strain varies spatially and temporally (minimum and maximum -10 and 4 %, respectively) with strain rate changing up to 100 %/s contraction and 120 %/s elongation. Most of the strain energy is related to annular axial strain. However, implantation of sonocrystals may alter the annulus deformation, in addition to differences in behavior among mammalian species.
continuity. Axial strain varies spatially and temporally (minimum and maximum -10 and 4 %, respectively) with strain rate changing up to 100 %/s contraction and 120 %/s elongation. Most of the strain energy is related to annular axial strain. However, implantation of sonocrystals may alter the annulus deformation, in addition to differences in behavior among mammalian species.
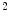 continuity. Axial strain varies spatially and temporally (minimum and maximum -10 and 4 %, respectively) with strain rate changing up to 100 %/s contraction and 120 %/s elongation. Most of the strain energy is related to annular axial strain. However, implantation of sonocrystals may alter the annulus deformation, in addition to differences in behavior among mammalian species.
continuity. Axial strain varies spatially and temporally (minimum and maximum -10 and 4 %, respectively) with strain rate changing up to 100 %/s contraction and 120 %/s elongation. Most of the strain energy is related to annular axial strain. However, implantation of sonocrystals may alter the annulus deformation, in addition to differences in behavior among mammalian species.Simulations were carried out to assess the stress field in the mitral valve anterior leaflet, using different pseudo-hyperelastic constitutive models, especially a collagen fiber-mapped transversely isotropic model, and a given valve microstructure [496]. However, the collagen fiber arrangement and material constant may not represent perfectly in vivo data.
6.3 Tricuspid Valve Structure
The tricuspid valve is composed of an anterior (anterosuperior, ventral, or mural), posterior (inferior or dorsal), and septal (or medial) leaflet. It builds a structural unit with the annulus, chordae tendinae, and papillary muscles. The latter vary in numbers (2–9; usually 3: anterior, posterior, and septal).
Its orifice is larger than that of the mitral valve. Its leaflets are thinner than those of the mitral valve.
The annulus area undergoes three-dimensional changes during the cardiac cycle. The annulus also forms a part of the triangle of Koch together with the coronary sinus and the tendon of Todaro that incorporates the nodal conduction tissue.
The three leaflets of the tricuspid valve differ in size. The anterior cusp is the largest. It spreads from the infundibular area downward to the inferolateral wall of the right ventricle. The septal leaflet is attached to both the membranous and muscular portions of the ventricular septum. The posterior cusp is the smallest. Sometimes, four leaflets can be identified; the posterior leaflet can be divided or an additional leaflet is positioned between the posterior and septal cusps. They are mainly composed of a fibrous skeleton and covered by an endocardium. The atrial layer of the endocardium is smooth. The lamina spongiosa is composed of loose layer of connective tissue and the lamina fibrosa of dense collagen fibers. The fibers are parallel. The innervation of human tricuspid valve leaflets is stronger than that of mitral leaflets.
Several chordae tendinae are attached directly to the interventricular septum. The chordae tendinae are interconnected before they attach the leaflet free margins. They are composed of a network of collagen fibers parallel to the axis.
6.4 Pulmonary Valve Structure
The pulmonary valve connects the conus arteriosus (infundibulum) of the right ventricular outflow tract to the pulmonary artery trunk. The pulmonary valve is part of the pulmonary root. Its associated structures include the sinuses (sinus trunci pulmonalis), annulus, commissures, leaflets, and sinotubular junction. The pulmonary leaflets are thinner and the noduli of Arantii smaller than those of the aortic leaflets [481].
The annulus of the pulmonary root is defined by the line of attachment of the leaflets to the sinus wall (crown-shaped annulus), as is the aortic annulus. It is composed of tight collagenous tissue. It is connected to the media of the pulmonary artery and the myocardium.
The commissures are the short parallel parts of attachment lines of the leaflets to the pulmonary wall. Three commissures exist between the left and right anterior leaflets, between the right anterior and posterior leaflet, and between the posterior and the left anterior leaflet.
The interleaflet triangles are the area under the commissures. They are extensions of the right ventricular outflow tract.
The three sinuses of Valsalva correspond to the left and right anterior and posterior leaflets. The sinus wall is much thinner than that of the pulmonary artery, especially in its middle portion.
The sinotubular junction of the pulmonary root is characterized by a ridge which defines the upper part of the sinuses and runs through the upper part of the commissures.
The leaflets of the pulmonary valve consist of the hinge, belly, coaptation surface, and lannula with the nodulus of Arantius, as those of the aortic valve. Collagen and elastin fibers serve as load-bearing components. Five layers exist between the ventricular and arterial endocardia (made up of endotheliocytes that interdigitate or overlap over a basement membrane) [481]:
The innervation arises from the ventricular endocardial plexus and localizes to the ventricular layer and lower region of each leaflet.
Lamina ventricularis (thickness 21–48 μm; with reticular fibers)
Lamina radialis (thickness 58–108 μm; with radial collagen and elastin fibers)
Lamina spongiosa (thickness 40–300 μm; with loosely arranged reticular fibers and bundles of collagen and some elastin fibers)
Lamina fibrosa (thickness 80–170 μm; with circular collagen fibers)
Lamina arterialis (very thin layer of reticular fibers)
6.5 Left-Sided Valve Failure
Valvular diseases are mostly due to either congenital abnormalities or inflammation. Primary valve failure occurs acutely due to leaflet perforation or gradually from leaflet stiffening associated with calcifications and/or thrombus formation.
Calcific vasculopathies can be distinguished by its histological appearance as amorphous mineralized medium, without tissular organization, or as chondrobony milieu, which have architecture of cartilage or bone, when angiogenesis occurs.
6.5.1 Mitral Valve Failure
The mitral cusps are dynamically related to the left ventricular wall via the mitral annulus, chordae tendineae, and papillary muscles. Mitral diseases may be degenerative (∼ 60 %), ischemic (25 %), infective (endocarditis), or rheumatic (12 %), with or without superimposed impaired left ventricular function and calcification.
When the mitral valve fails, partial emptying of the left atrium increases the left arterial pressure and reduces the cardiac flow rate. Pulmonary venous congestion and, ultimately, pulmonary edema occur.
A systematic analysis of the anomalies of the valvular structure and function is mandatory for selecting relevant therapeutic actions. Mitral regurgitation results from either leaflet perforation, such as that due to trauma or endocarditits, or annular dilation, usually due to left ventricular damage.
Three types are defined in the Carpentier’s classification of mitral valve dysfunction (Table 6.4). The Carpentier’s functional classification describes leaflet motion in relation to the mitral annular plane:
Type 1
corresponds to a normal leaflet motion.
Type 2
describes excessive leaflet motion above the annular plane into the left atrium. This cusp prolapse is usually caused by tissular degeneration.
Type 3
accounts for leaflet restriction. Two subtypes are defined: type-3a when the restriction is throughout the cardiac cycle and type-3b, when the leaflet restriction occurs only during systole.
The mitral valve prolapse is defined by leaflets extending above the plane of the atrioventricular junction during ventricular systole. It can result from several mechanisms, such as rupture of cords and tips of papillary muscles and abnormal wall motion secondary to myocardial ischemia. The primary prolapse is a floppy valve that domes into the left atrium and, often, with heterogeneously deformed and thickened cusps.
Two forms of prolapse are identified: (1) The leaflets protrude into the atrium, but they can coapt, ensuring valvular competence. (2) The free edge of the damaged leaflet overshoots the closure line of the opposite leaflet, thereby causing mitral regurgitation.
Table 6.4
Carpentier’s classification of mitral valve dysfunction
Dysfunction | Lesions |
|---|---|
Leaflet motion | |
Type 1: | Annular dilation |
Normal motion | Leaflet perforation |
Central regurgitation jet | |
Type 2: | Chordal rupture or elongation |
Excess leaflet motion | Papillary muscle rupture or elongation |
(prolapse) | |
Eccentric regurgitation jet | |
Type 3: | Commissural fusion |
Restricted leaflet motion | Chordal fusion and thickening |
Valve thickening and/or calcification | |
Eccentric regurgitation jet | |
6.5.1.1 Mitral Valve Stenosis
Mitral valve stenosis (MVS) impedes adequate blood flow coming from the left atrium to the left ventricle. The resulting obstruction causes a pressure difference across the valve in diastole and increases left atrial and pulmonary venous pressures. Elevated left atrial pressures lead to left atrial enlargement and predispose to atrial fibrillation and arterial thromboembolism. Upstream blood stasis can engender pulmonary congestion and edema. Evolved mitral stenosis is associated with pulmonary hypertension and right-heart failure.
The main cause of mitral valve stenosis is rheumatic fever following a Streptococcus pyogenes infection, such as streptococcal pharyngitis. Myocarditis can lead to congestive heart failure with pericarditis. About half of patients with acute rheumatic fever develop inflammation of valvular endothelium.
Rheumatic valvulitis results from an immune reaction to group-A β-hemolytic streptococci that have antigens immunologically related to the human heart. The cross-reactive autoimmunity can be stimulated in some persons after streptococcal infection. CD4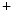 T lymphocytes activated by molecular mimicry are the major effectors of cardiac autoimmune reactions.
T lymphocytes activated by molecular mimicry are the major effectors of cardiac autoimmune reactions.
< div class='tao-gold-member'>
 T lymphocytes activated by molecular mimicry are the major effectors of cardiac autoimmune reactions.
T lymphocytes activated by molecular mimicry are the major effectors of cardiac autoimmune reactions.Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


