Invasive procedures
Noninvasive methods
Intracoronary injection of tyramine
Invasive evaluation of the response to exercise
Radiolabeled catecholamine analogues
Radiolabeled receptor antagonist
Guanethidine analogue 123I-MIBG
Catecholamine analogue ¹¹C-HED
β-Adrenergic receptor antagonist ¹¹C-CGP-12177
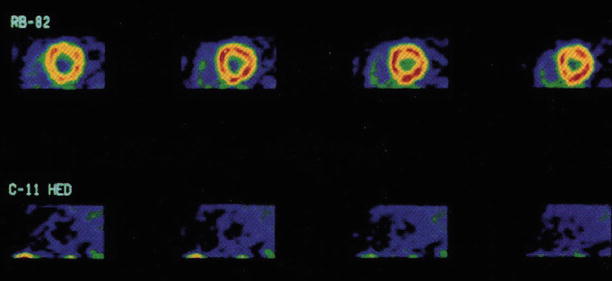
Figure 10-1.
Positron emission tomography (PET) imaging with the catecholamine analogue ¹¹C-HED may be used to trace and quantitate norepinephrine uptake and storage in the presynaptic adrenergic nerve terminals [14–18]. Shown here are cross-sectional PET images from a patient with recent cardiac transplantation: rubidium-82 (Rb-82) presents homogeneous blood flow. Myocardial retention of ¹¹C-HED 30 min after tracer injection is markedly reduced compared with that of Rb-82. A PET tracer that can image cardiac β-adrenergic receptor density also is available in the form of ¹¹C-CGP-12177, a β-adrenergic receptor antagonist [19–21]. Similarly, PET imaging of parasympathetic (muscarinic) nerve function also has been used in evaluating the extent and time course of parasympathetic reinnervation of the transplanted heart [22, 23] (From Schwaiger et al. [18]; with permission).
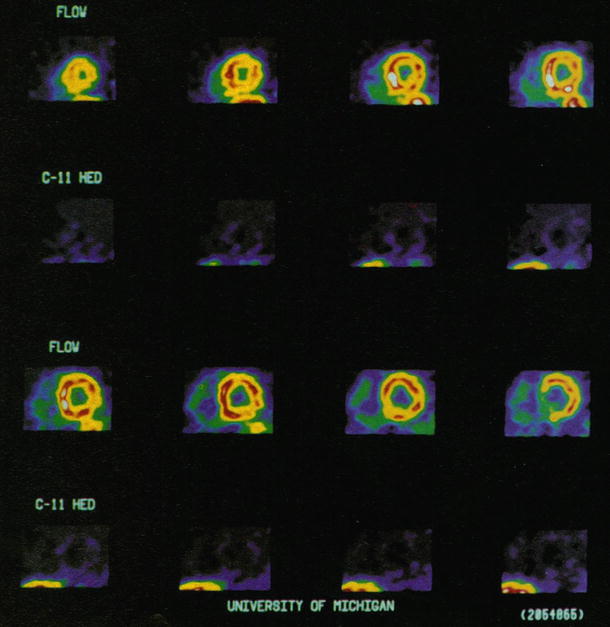
Figure 10-2.
Most studies have confirmed complete denervation within the first 6–12 months after transplantation. Show here are ¹¹C-HED and Rb-82 short-axis PET images obtained from a patient with recent cardiac transplant (3 months after cardiac transplantation). The blood flow images show homogeneous blood flow throughout the left ventricle, whereas the ¹¹C-HED images below indicate a marked reduction of tracer retention that is homogeneous throughout the left ventricle (From Schwaiger et al. [14]; with permission).
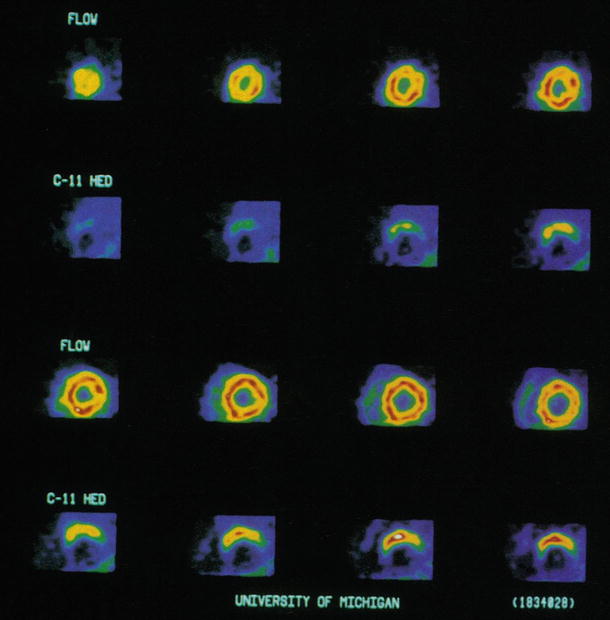
Figure 10-3.
Myocardial adrenergic reinnervation after heart transplantation is a progressive process with a definitive pattern that usually starts during the second year after transplantation [8–18, 24, 25]. Shown here are short-axis PET images obtained in a patient 55 months after cardiac transplantation. Rb-82 images show homogeneous blood flow throughout the left ventricle. ¹¹C-HED images below indicate tracer retention in the anterior aspects of the left ventricle, increasing from the mid–left ventricle toward the base of the left ventricle (bottom row) (From Schwaiger et al. [14]; with permission).
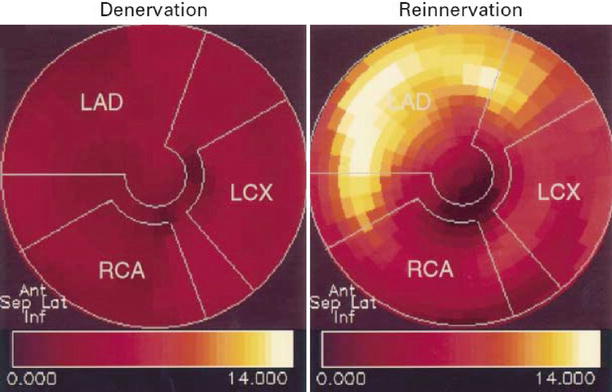
Figure 10-4.
The first evidence of myocardial sympathetic reinnervation is found in basal parts of the anterior (Ant) wall and subsequently progresses to more distal parts of the myocardium. In addition to the gradient from base to apex, the anterior and septal (Sep) walls are reinnervated earlier, whereas the lateral (Lat) wall appears to be involved later. The polar maps show the level of ¹¹C-HED retention (percent per minute) in one patient with denervation and one with reinnervation. The apex is in the center, the base in the periphery, the anterior wall on top, the septum on the left, the lateral wall on the right, and the inferior (Inf) wall on the bottom. The patient with denervation has low levels of ¹¹C-HED retention in all regions (dark red), whereas the patient with reinnervation has a high level of ¹¹C-HED retention (yellow) in the basal anteroseptal wall, indicating partial restoration of catecholamine uptake sites. LAD left anterior descending artery, LCX left circumflex artery, RCA right coronary artery (From Bengel et al. [24]; with permission).
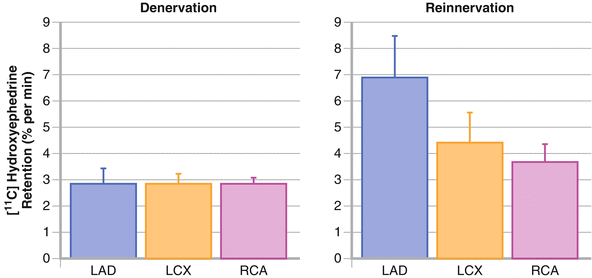
Figure 10-5.
The myocardial sympathetic reinnervation process, particularly that involving the anterior and, to a lesser extent, the lateral LV wall, suggests that sympathetic nerves are first restored in the territory of the LAD artery followed later by the LCX territory. In contrast, the territory of the RCA demonstrates little or no reinnervation until late after transplant, when there is only modest, at most, evidence of sympathetic nerve terminals at the apex and inferior wall of the left ventricle [10, 13, 15, 17, 24] (Adapted from Bengal et al. [24]).
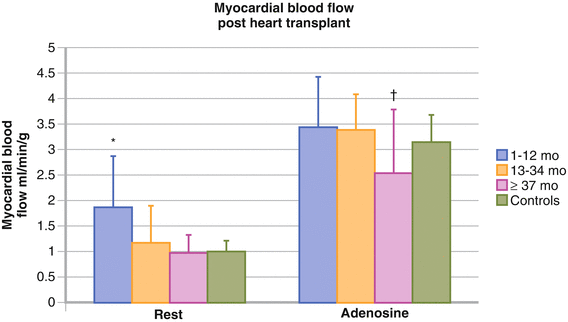
Figure 10-6.
Myocardial blood flow after heart transplantation may be quantified by PET imaging with nitrogen-13 (¹³N)-ammonia or H2 15O [21, 26]. Myocardial blood flow at rest and with adenosine is shown for different patient groups according to the interval after heart transplantation, as well as for a healthy control group. Under resting conditions, myocardial blood flow increased in the 1- to 12-month group versus the other groups. Increases in myocardial blood flow in response to adenosine were comparable among transplant recipients 1 and 36 months after transplantation and did not differ significantly from the control group. In contrast, myocardial blood flow with adenosine was reduced in the ≥37-month group versus the other groups. This finding suggests that an immune-mediated mechanism may cause endothelial dysfunction and impairment of microvascular dilator function as posttransplant time increases beyond 3 years. Values are expressed as mean; Error bars represent SD. *P < 0.02 for the 1- to 12-month group versus all other groups. †P < 0.05 for the ≥37-month group versus the 1- to 12-month and 13- to 34-month groups, and P = 0.06 versus controls. (Adapted from Kushwaha et al. [27]).
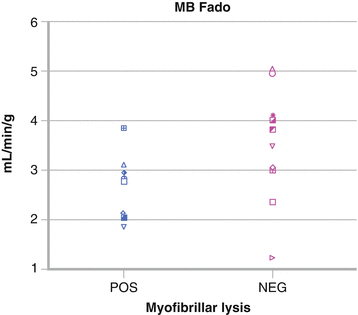
Figure 10-7.
Myofibrillar lysis correlated strongly with reduced maximal dilator capacity in response to adenosine (<3 mL/min/g) in all transplant recipients regardless of time interval from transplantation. It appears likely that an immune-mediated mechanism causes both the myofibrillar injury and the impairment of microvascular dilator function [27]. Shown here are individual data values of maximal myocardial blood flow with adenosine (MBFado) for patients after heart transplantation according to the presence (POS) or absence (NEG) of myofibrillar lysis on biopsy. In general, patients with myofibrillar lysis have a maximal myocardial blood flow <3 mL/min/g, whereas those without it have a maximal myocardial blood flow >3 mL/min/g (Adapted from Kushwaha et al. [27]).
Table 10-2.
The effect of reinnervation on heart rate response during exercise after cardiac transplantation.
Normal control | Transplant recipients <6 m | Transplant recipients >1 y | |||
|---|---|---|---|---|---|
Denervated | Mild–moderate reinnervation | Marked reinnervation | |||
Basal HR (bpm) | 76 ± 3a | 88 ± 2 | 85 ± 3 | 94 ± 4 | 87 ± 3 |
HR at anaerobic threshold (bpm) | 151 ± 3a | 109 ± 4 | 106 ± 5 | 121 ± 9b | 120 ± 3b |
HR at peak exercise (bmp) | 178 ± 4a | 129 ± 3 | 126 ± 5 | 142 ± 7 | 141 ± 2b |
Recovery HR (% of peak HR) | 64 ± 3a | 94 ± 1 | 91 ± 1 | 87 ± 3b | 80 ± 2b |
Change in HR after tyramine injection (bpm) | 1.6 ± 0.3 | <5 | 5–14 | ≥ 15b | |



