Cardiac Transplantation
Anantharam V. Kalya
Jeffrey David Hosenpud
Over the past 30 years, cardiac transplantation has evolved from a highly experimental procedure performed in a handful of centers to an accepted modality of therapy for the treatment of end-stage heart disease. Cardiac transplantation is now performed on every continent and in over 300 centers worldwide (1). Unfortunately, despite continuing expansion of the criteria for acceptable donor organs, the availability of donor hearts limits the availability of this form of therapy. It is estimated that in the United States alone, over 20,000 patients could benefit from cardiac transplantation yet the number of donor hearts procured is only around 2,500 (2). This chapter reviews the current state of cardiac transplantation, its successes, and the challenges yet to be overcome.
Recipient Selection
The basic tenets expressed in the criteria developed by the Stanford group in the early 1970s (3,4) continue to apply today. Cardiac transplantation must be reserved for those patients with disabling symptoms of congestive heart failure (New York Heart Association [NYHA] late functional Class III and IV) whose likelihood of survival is poor over the next 6 to 12 months. There have been, however, several modifications of ancillary inclusion and exclusion criteria over the past 20 years. As a result, cardiac transplantation is now being offered to sicker and higher-risk patients. Table 46-1 outlines the inclusion and exclusion criteria generally agreed-upon by the transplant community. In addition, given the shortage of acceptable organs for heart transplantation, attempts are currently under way to further refine these criteria and ultimately establish uniform listing criteria. One such attempt by the United Network for Organ Sharing (UNOS) has proposed the criteria shown in Table 46-2. Other groups, including the American Heart Association (AHA), have published position papers regarding appropriate listing criteria (5,6).
Age
Based on early data from Stanford showing a 20% decrement in 1-year survival in patients over the age of 50 years, the upper age limit for cardiac transplantation was considered 50 years (3). Several subsequent single-center studies could not demonstrate a mortality increase in patients between the ages of 50 and 65 (7,8,9). One additional study investigating not only survival, infection, and rejection but also overall hospitalization and noncardiac morbidity demonstrated no major differences in these parameters between those patients above and below the age of 55 (10). A more recent study performing similar analyses in patients above and below the age of 65 did, however, demonstrate that those over age 65 had prolonged hospitalizations, longer rehabilitation, and a trend toward reduced survival that did not reach statistical significance (11).
These single-center experiences have not been confirmed by multicenter or registry data (12). Using multivariate analysis, the joint UNOS/International Society for Heart and Lung Transplantation (ISHLT) Thoracic Registry has consistently shown an independent, linear increased risk with increasing age that is highly statistically significant (1). Despite these data, the standards in the transplant community are to offer heart transplantation up to the age of 65 years, with rare individuals (approximately 2% of all patients) transplanted over the age of 65.
Table 46-1 Recipient Selection Criteria | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Pulmonary Vascular Resistance
Severe and irreversible (nonreflex) pulmonary hypertension has consistently been demonstrated to be a risk factor for poor outcome following cardiac transplantation in both single-center (3,13) and multicenter (14) reports. The reason for this is the inability of the transplanted right ventricle to acutely adapt to elevated pulmonary artery pressures. The transplanted right ventricle is usually procured from a donor with normal pulmonary vascular resistance (PVR) and, hence, is of normal thickness. In addition, it is extremely susceptible to preservation injury and perioperative dysfunction from rapid rewarming during the implantation procedure. Potential recipients who have pulmonary artery hypertension on routine cardiac catheterization (a PVR of greater than 3 Wood units) receive intravenous vasodilators (sodium nitroprusside, prostaglandin E, nitric oxide by infusion/inhalation in gradually increasing doses to blood pressure tolerance) to determine whether the elevation in pulmonary pressures is secondary to pulmonary vasoconstriction or irreversible disease.
Frequently, pulmonary artery pressures and resistance will fall into the acceptable range with acute pharmacologic intervention. A small number of patients, however, with truly reversible pulmonary hypertension will require several days of intensive medical management before
pulmonary pressures fall. We have observed that selected patients on left ventricular assist devices (LVADs), who had severe pulmonary hypertension and suboptimal response to vasodilators, show significant improvement in their pulmonary pressures and vascular resistance after a few months of LVAD support (15). Those patients who persist in having elevated PVR despite intensive medical management may be candidates for heart-lung transplantation.
pulmonary pressures fall. We have observed that selected patients on left ventricular assist devices (LVADs), who had severe pulmonary hypertension and suboptimal response to vasodilators, show significant improvement in their pulmonary pressures and vascular resistance after a few months of LVAD support (15). Those patients who persist in having elevated PVR despite intensive medical management may be candidates for heart-lung transplantation.
Table 46-2 Listing Criteria Proposed by the United Network for Organ Sharing (Unos) Subcommittee on Uniform Listing Criteria | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Infection
Patients in severe congestive heart failure are at increased susceptibility to infection, especially those waiting in intensive care units for cardiac transplantation. Because of the immediate requirements for immunosuppression following cardiac transplantation, patients must be adequately treated for any recent infection and be free of infection at the time of transplantation. In general, upper respiratory viral infections and uncomplicated bacteria have not been considered a contraindication for proceeding to transplantation.
Noncardiac Organ System Dysfunction
Severe congestive heart failure is frequently associated with prerenal azotemia and passive hepatic congestion (16,17). It is therefore important to separate these abnormalities from intrinsic organ dysfunction, as several of the commonly used immunosuppressive agents have either renal or hepatic toxicity (18,19). Patients with a serum creatinine greater than 2 mg/dL or hepatic enzyme abnormalities greater than twice normal should therefore have careful evaluations to exclude intrinsic organ dysfunction. In addition, severe heart failure frequently impacts pulmonary function. In a series of 17 patients studied with spirometry before and several months following cardiac transplantation, the principal spirometric abnormality was a reduction in lung volumes (restrictive pattern) before transplantation that was completely reversible following transplantation (20). The reduction in lung volumes was strongly correlated to the increase in cardiac volume, and obstructive physiology (if present before transplantation) was unchanged following cardiac replacement and normalization of hemodynamics. These data suggest that severe obstructive pulmonary physiology before transplantation would be unlikely to improve substantially following cardiac transplantation. Therefore, those patients with a forced vital capacity less than 50% of predicted with obstructive physiology would be extremely high-risk for postoperative pulmonary complications and nosocomial infection.
Systemic Diseases and Prior Malignancies
Diabetes had initially been considered a contraindication to cardiac transplantation because of the corticosteroids required as part of the immunosuppressive regimen. With the advent of lower-dose steroid protocols utilizing cyclosporine-based immunosuppression, the inclusion of diabetics as candidates for cardiac transplantation has gradually increased. Diabetics with severe end-organ damage are still, for the most part, excluded as candidates. Otherwise, it appears that patients with controlled diabetes have acceptable outcomes following cardiac transplantation (21).
Although definitive studies have not been performed, patients with prior malignancies who are considered cured are now being considered for and have undergone cardiac transplantation (22). The disease-free interval that is acceptable is obviously highly dependent on the natural history of the underlying malignancy. Several of these patients were treated with doxorubicin and have cardiomyopathy and heart failure on this basis (23). Whether these patients are at a higher risk for the development of recurrent or new malignancies is yet to be determined.
Finally, other systemic diseases such as amyloidosis and sarcoidosis have been traditionally considered contraindications for cardiac transplantation because of the concern that these would recur in the allografted organ. The initial small experience with amyloidosis appeared to be positive, with seven patients having intermediate-term survival equivalent of that of an age- and sex-matched control group (22). A longer follow-up of these patients demonstrated that despite the favorable intermediate prognosis, most patients developed progressive amyloid involvement in major organ systems and ultimately a reduced survival (24). Anecdotal reports of patients with cardiac sarcoidosis undergoing cardiac transplantation are available (25,26). Sarcoid granulomas can, however, recur in the allograft (27).
Medical Compliance, Substance Abuse, and Psychosocial Support
The complexity of the pre-, peri-, and post-operative care in cardiac transplantation necessitates a patient who understands the disease and is willing to comply with the recommendations made by the transplant team. Active substance abuse clearly threatens this compliance. The potential emotional stress in all aspects before and after transplantation may be better coped with if social support is available for the patient. Interestingly, however, it is extremely difficult to predict medical outcome and compliance using standard psychosocial evaluation parameters (28).
Recipient Evaluation
Table 46-3 outlines the typical evaluation performed for a patient referred for cardiac transplantation. The principal goals of this evaluation are (a) to determine the underlying cardiac disease and, if possible, delineate alternative treatment strategies; (b) to quantify cardiovascular function, degree of symptoms attributable to the cardiac function, and PVR; (c) to determine whether immunological barriers, such as preformed antibodies to histocompatibility leukocyte antigens (HLAs), are present, either precluding transplantation or modifying posttransplant treatment approaches; and (d) to evaluate other organ function and disease that might impact posttransplant outcome.
Table 46-3 Recipient Evaluation | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The Cardiac Donor
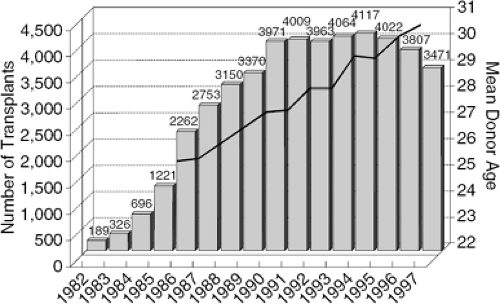
The passage of the Uniform Anatomical Gift Act in 1968 (29), the general acceptance of criteria for brain death (30), and an increasing public awareness have brought about an increase in the number of available organs. However, in the past several years the number of donors and heart transplant operations worldwide has reached a plateau despite expansion of the criteria for an acceptable heart donor, consistent with a reduction in the numbers of what was previously considered the optimal heart donor. This, coupled with expanded recipient criteria, has resulted in a greater donor-recipient number mismatch. Figure 46-1 demonstrates the increase in donor age that has had to be required to maintain the relatively consistent number of transplants being performed worldwide (1). In 1988, the median waiting time for heart transplant candidates was 117 days. In 1996, the time to transplant had increased to 224 days, almost double (2). The median waiting time for heart transplant in 1999 was 502 days, and in 2002 was 392 days (UNOS Organ Procurement and Transplantation Network: Median waiting times for registrations listed between 1997–2002). The reduction in the heart transplant listings from 1999 to 2002 may reflect changes in listing criteria (31). The American College of Cardiologists/American Heart Association (ACC/AHA) guidelines for heart failure management have been more stringent regarding the listing criteria because of improved survival among the heart failure patients on medical management (32).
Brain Death
Neither the organ procurement agency nor the transplant teams have any involvement with the patient until brain death is declared and consent for organ donation is obtained. Table 46-4 presents the clinical and laboratory findings in brain death. In general, there is absence of cortical function as assessed by spontaneous movement, response to stimuli, or response to pain. There is absence of brainstem function, including a host of brainstem reflexes and spontaneous respiration. Finally, the electroencephalogram has no activity and, if measured, there is no cerebral blood flow (33,34).
Table 46-4 Brain Death | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Screening for Organ Donation
Aspects of screening of potential donors can be divided into those required for screening the donor for any and all organ donation and those specific for cardiac donation. Generalized donor screening centers around excluding transmissible diseases such as infections and malignancy. Screening now routinely carried out includes a careful medical and social history to eliminate patients whose exposure or lifestyles might increase the likelihood of viral diseases such as hepatitis B or C or human immunodeficiency virus (HIV); specific serology for hepatitis B and C, HIV, and human T-cell lymphotropic virus 1 (HTLV-1); and surveillance bacterial cultures. Patients with active sepsis are usually not considered for organ donation. Localized infection in the lung or urinary tract may allow for the donation of organs not directly involved. Likewise, despite malignancy being a contraindication for organ donation, patients with localized central nervous system malignancy are generally considered acceptable organ donors.
Once the potential organ donor has been screened for the general contraindications to organ donation, specific issues dealing with cardiac donation can then be addressed. The issues relate primarily to two questions: the function of the heart and the possibility of occult or overt coronary disease. Initially, cardiac donors were strictly age-limited to men aged 30 years or less and women aged 35 years or less, specifically to reduce the likelihood of occult coronary disease (35). With the progressive shortage of donors, this age limit was increased to 40 and 45 years, respectively (36); currently, many centers will consider organ donors age 50 years and beyond, depending on the age and stability of their recipients. The use of an older donor will, however, have an impact on overall posttransplant survival, given the known increase in mortality of the recipient as donor age increases (12). In general, most centers will attempt to obtain coronary angiography on the older donors but in some smaller hospitals this is not always achievable. With the same concerns, insulin-requiring diabetics are generally excluded from cardiac donation unless coronary angiography can be obtained, and those with other coronary risk factors are carefully evaluated. The electrocardiogram can be extremely helpful in this regard if pathological Q waves are present. ST segment shifts, T-wave changes, and arrhythmias are generally not helpful, as all of these are not infrequently associated with brainstem herniation and brain death (37,38).
The first indication of the integrity of cardiac function is the degree of inotropic support required to maintain stable vital signs in the donor. This, however, can be extremely misleading because, before brain death, a goal of the physicians (neurosurgeons or neurologists) caring for the patient is to minimize cerebral edema. This is usually accomplished by aggressive diuresis and fluid restriction. It is not unusual to find a potential organ donor receiving high-dose inotropic and vasopressor support, which can be rapidly weaned with aggressive fluid replacement. Most cardiac transplant programs will further evaluate a potential donor if inotropic support (usually either dobutamine or dopamine) can be reduced to below 10 μg/kg per minute. The echocardiogram (ECG) is now frequently utilized to evaluate cardiac function in the donor. Gilbert et al. demonstrated that echocardiography identified a full 29% of their successful cardiac donors who would have otherwise been excluded using ECG and clinical findings alone (39). Table 46-5 reviews the current screening criteria for cardiac organ donation.
Table 46-5 Screening Criteria for Cardiac Donation | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The Transplant Operation
The cardiac transplant operation is, in fact, a multifaceted procedure that involves two operations—myocardial protection and organ transportation. With some modifications, the technical aspects of the cardiac transplant operation are essentially those initially described by Lower and Shumway 45 years ago (40).
Myocardial Preservation
The techniques of cold cardioplegia have been developed in cardiovascular surgery over the past 20 years. There appears to be adequate preservation of the myocardium at temperatures below 20°C. A number of preservation solutions have been employed over the years, with centers gradually moving away from using standard Euro-Collins solution or Stanford University solution to newer solutions such as University of Wisconsin (UW) solution. In a recent survey performed by the University Hospital Consortium, 39% of centers were using Stanford solution, 22% UW solution, and the remainder a variety of preservation solutions. The UW solution has been demonstrated to have a profound effect on both renal and liver preservation for transplantation. Although there are animal studies demonstrating improved cardiac preservation with UW solution (41,42), recent animal studies and one clinical study suggest that UW solution is associated with endothelial cell injury and a higher incidence of allograft vasculopathy (chronic rejection) posttransplantation (42,43).
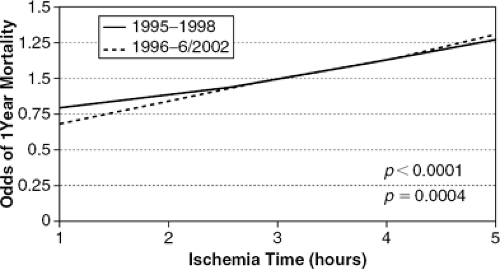
Ischemic Time
As shown in Figure 46-2 based on data from the joint UNOS/ISHLT Thoracic Registry, it is absolutely clear that ischemic time (the time from aortic cross-clamp in the donor to cross-clamp release and restoration of coronary flow in the recipient) is a strong and independent determinant of early survival (12,44,45). Most centers will, therefore, not exceed 4 hours of ischemic time unless their recipient is extremely unstable. Unfortunately, it is the sicker recipients who generally need a well-functioning allograft in the early posttransplant period. Methods for improving myocardial preservation, thus allowing for greater ischemic times, are currently under active investigation. One would hope that with improved preservation and better immunological matching of donors and recipients, greater use of donated cardiac organs could be achieved.
Traditional (Shumway) Donor Cardiectomy
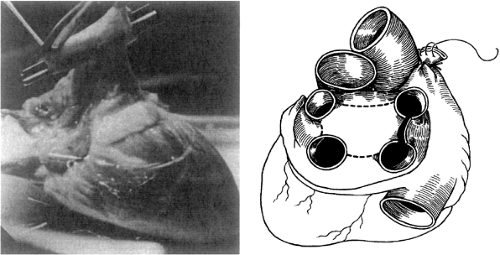
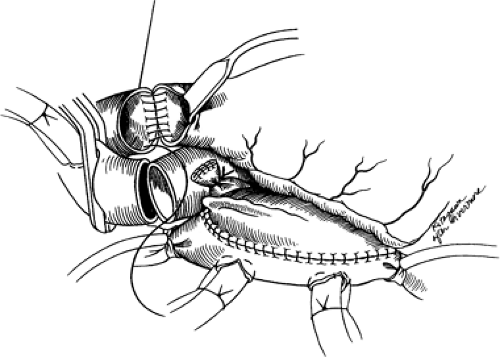
The donor heart is reached through a standard midline sternotomy. The heart is inspected visually to evaluate chamber size and wall motion and palpably to detect the degree of chamber filling and the presence of coronary calcification. Once the heart is considered appropriate for transplantation, the transplant center is notified and the recipient operation begins. While other organs are being harvested, the aorta and pulmonary artery are dissected free and controlled with tapes, as is the superior vena cava. Just before harvest, heparin is administered, the superior vena cava is ligated, the inferior vena cava is cross-clamped, and cold cardioplegic solution is administered into the aorta. The aorta is then cross-clamped and the various cardiac structures are transected in the following order: (a) the superior vena cava (between double ligations); (b) inferior vena cava and right lower pulmonary vein; (c) aorta and pulmonary artery; and finally, (d) the remaining pulmonary veins. The pulmonary veins are connected to form the left atrial cuff and the heart is packaged for transport. Figure 46-3 demonstrates the preparation of the donor heart.
Traditional (Shumway) Recipient Operation
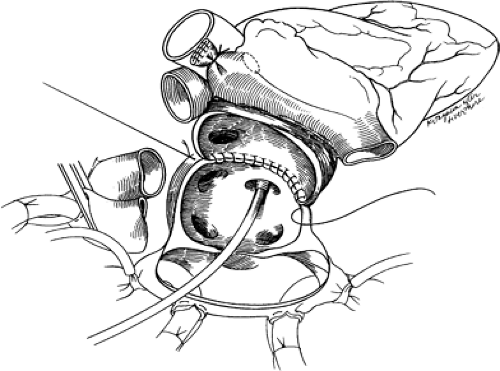
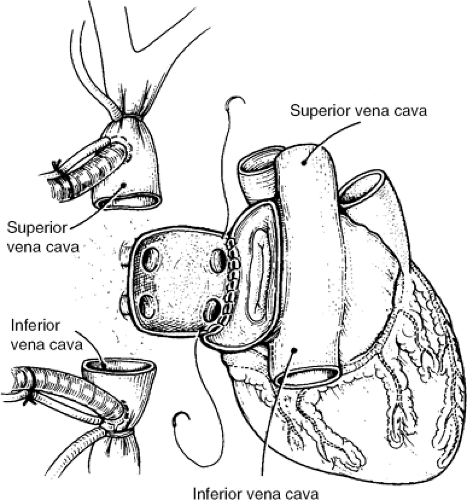
The recipient is brought to the operating room and central venous (or pulmonary artery) and intra-arterial catheters are placed. The operation is via a midline sternotomy, usually started when communication from the donor team indicates the donor heart is suitable for transplantation. After dissection and control of the great veins and arteries, heparin is administered and the patient is cooled and placed on cardiopulmonary bypass (usually not before the donor heart has arrived at the transplant center). The native heart is excised with incisions transecting the various cardiac structures in the following order: (a) the lateral wall of the right atrium, continuing inferiorly and medially to and across the intra-atrial septum; (b) the aorta and pulmonary artery; and, finally, (c) the left lateral wall of the left atrium. The allograft implantation is then carried out using running suture with anastomoses in the following order: (a) left atrial free wall, (b) intra-atrial septum, (c) right atrium, (d) pulmonary artery, and (e) aorta. The early and late stages of the allograft implantation are demonstrated in Figures 46-4 and 46-5, respectively.
Bicaval/Total Recipient Operation
Two more recent variations of this procedure have been undertaken to attempt to reduce injury to the sinus node and to improve atrial transport by avoiding the competing atrial contractions between the recipient and donor atria. The initial bicaval anastomotic approach is shown in Figure 46-6. In this procedure, the recipient right atrium is
completely removed and the inferior and superior vena cavae are anastomosed directly to the intact donor atrium. The anastomosis of the left atrium is similar to the traditional approach. A modification this approach described by Dreyfus et al. (46) is to remove the recipient left atrium, leaving only left and right pulmonary vein “buttons,” which are directly anastomosed to the intact donor left atrium (not shown). In a small study involving 18 patients, Sievers et al. demonstrated the preservation in atrial size and a reduction in tricuspid regurgitation during exercise in those undergoing the bicaval approach versus the traditional approach, but no difference in exercise capacity could be demonstrated (47). Deleuze et al., in a much larger series, demonstrated that those patients undergoing the newer technique had less delayed sinus node function and initially lower pulmonary artery pressure and higher resting cardiac indeces compared to those undergoing the traditional anastomosis (48). Whether long-term outcomes are different is yet to be determined.
completely removed and the inferior and superior vena cavae are anastomosed directly to the intact donor atrium. The anastomosis of the left atrium is similar to the traditional approach. A modification this approach described by Dreyfus et al. (46) is to remove the recipient left atrium, leaving only left and right pulmonary vein “buttons,” which are directly anastomosed to the intact donor left atrium (not shown). In a small study involving 18 patients, Sievers et al. demonstrated the preservation in atrial size and a reduction in tricuspid regurgitation during exercise in those undergoing the bicaval approach versus the traditional approach, but no difference in exercise capacity could be demonstrated (47). Deleuze et al., in a much larger series, demonstrated that those patients undergoing the newer technique had less delayed sinus node function and initially lower pulmonary artery pressure and higher resting cardiac indeces compared to those undergoing the traditional anastomosis (48). Whether long-term outcomes are different is yet to be determined.
Perioperative Care
Immunosuppression Induction
Although immunosuppression is discussed in depth later in this chapter, a few comments are appropriate at this time. The majority of centers continue to use cyclosporine (4 to 10 mg/kg) and azathioprine (2 to 5 mg/kg) preoperatively and intravenous prednisolone (0.5 to 2 g) intraoperatively. Some centers are using mycophenolate mofetil in place of azathioprine and/or tacrolimus in place of cyclosporine. In contrast to renal transplantation, the use of monoclonal and polyclonal anti-T-cell antibodies routinely as prophylaxis has declined over the past 5 years, primarily because of reports demonstrating an increased rate of infection and malignancy posttransplantation (49,50) and the relative lack of data suggesting improvement in outcomes using these agents (51,52). Monoclonal antibodies, specifically the monoclonal antibody to the T-cell receptor (anti-CD3) OKT3, have been effective in preventing
rejection in the first 14 days if cyclosporine has to be withheld because of early renal dysfunction. Newer monoclonal antibodies such as daclizumab (Zenapax), which is directed against the interleukin-2 (IL-2) receptor, may be similarly efficacious without some of the toxicity associated with OKT3. Polyclonal antibody preparations can also be used in this manner.
rejection in the first 14 days if cyclosporine has to be withheld because of early renal dysfunction. Newer monoclonal antibodies such as daclizumab (Zenapax), which is directed against the interleukin-2 (IL-2) receptor, may be similarly efficacious without some of the toxicity associated with OKT3. Polyclonal antibody preparations can also be used in this manner.
Allograft Hemodynamic Support
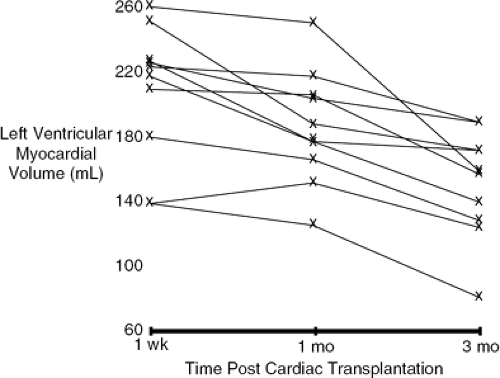
It is critically important to understand several aspects of allograft physiology early posttransplantation to provide appropriate postoperative support. Despite careful attempts at myocardial preservation and limiting total ischemic times, the allograft is ischemically damaged immediately following transplantation. This, coupled with mild to moderate elevations in PVR in the recipient (53), the denervated state of the allograft (54,55), and the ischemic damage to the sinus node (56), results in the heart and predominantly the right ventricle being in need of support. As one might anticipate, acute right ventricular failure is the most common nonimmunologic cause of perioperative death following cardiac transplantation (57). The ischemic damage to the allograft, in addition to being manifest as reduced systolic function and sinus node activity, is also manifest by a reduction in ventricular compliance associated with myocardial edema, which can be present for up to 3 months following transplantation (58,59).
Figure 46-7 demonstrates that left ventricular myocardial volumes measured by echocardiogram in 10 allograft recipients are still not normal by 1 month following transplantation. Stinson et al. investigated hemodynamics in 10 allograft recipients immediately postoperatively and for the first 7 days (60). Cardiac function was severely depressed initially (cardiac index 1.8 L/min/m2, stroke volume index 21 mL/m2) and then gradually improved. Isoproterenol was extremely effective in providing both inotropic as well as chronotropic support and is now, in most centers, the initial catecholamine of choice following transplantation. If additional inotropic support is required, it is important to choose agents that directly stimulate β1-receptors such as dobutamine or epinephrine or inhibit cyclic adenosine monophosphate (cAMP) breakdown by phosphodiesterase (milrinone, amrinone). Agents that indirectly provide inotropic support by releasing stored norepinephrine (or dopamine) will be effective for the first several hours following transplantation but will lose their effectiveness because of the denervated state. Elevations in PVR exacerbated by the use of blood products can be treated using vasodilators such as sodium nitroprusside or prostaglandin E2 (61). Most often, inotropic, chronotropic, and vasodilator support can successfully be discontinued between 3 and 5 days following transplantation.
Infection Control
Although early experience had most transplant centers using some form of protective isolation, the efficacy of these measures has never been demonstrated. As a result, the general trend has been to relax these measures to a considerable degree. Most patients receive prophylactic antibacterial agents effective against skin organisms for several days following transplantation. At centers where Pneumocystis and cytomegalovirus infections have produced substantial morbidity, patients receive prophylactic hyperimmune globulin and/or ganciclovir or acyclovir (CMV) and trimethoprim-sulfamethoxazole (Pneumocystis). The efficacy of these measures, although suggestive, is not clearly proven (62,63,64).
Immunosuppression: The Diagnosis and Treatment of Rejection
It is clear to all involved in the field of heart transplantation that with the advances in surgical care, the procedure itself is now only a minor part of the therapy of transplantation. The immunologic barrier between donor and recipient continues to be a substantial challenge. Table 46-6 presents the classification, mechanisms, and manifestations of the various forms of rejection. Hyperacute rejection is secondary to preformed alloantibodies, which are routinely screened for in the initial evaluation (previously discussed). Acute cellular rejection is the focus of the current discussion. Chronic rejection is manifest by an accelerated development of coronary artery disease (CAD), discussed later.
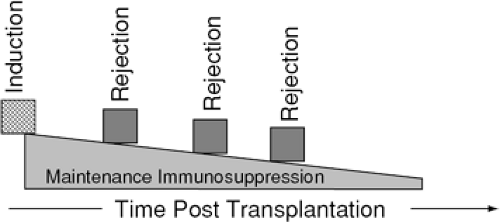
The primary concept of immunosuppression therapy is diagrammed in Figure 46-8. One initially induces an immunosuppressive state. Once induced, immunosuppression intensity is reduced over time, with episodic intensification for isolated episodes of rejection. Figure 46-9 demonstrates a simplified representation of the cellular immune response to alloantigens and the effects on this response by various classes of immunosuppressive agents. As stated previously, the specific immunosuppression protocols used vary substantially from center to center, but most use a combination of agents. Table 46-7 lists the specific agents by class. What follows is a brief description of the more commonly used agents.
Cyclosporine
This agent has clearly revolutionized the field of transplantation in its 15 years of clinical use. First discovered in 1972 by Jean Borel (65,66) and recognized as a potent inhibitor of T-cell function, it is now the mainstay of cardiac transplant immunosuppression. Cyclosporine is a T-cell-specific drug that inhibits the gene transcription of multiple lymphokines, including interferon-γ, interleukin-2 (IL-2), and probably interleukins 3 through 7 (67,68). These cytokines are extremely important for activation and recruitment of the various components of the immune system. For example, IL-2 is the primary signal for T-cell proliferation and clonal expansion (69). Interferon-γ may be primarily responsible for increasing target cell surface expression of major histocompatibility antigens necessary for recognition by the immune system (68). In clinical use, in most instances, a loading dose of between 5 and 10 mg/kg is administered preoperatively, and the maintenance dose ranges from 3 to 8 mg/kg in divided doses. The specific dose for a given patient is determined by blood or serum levels and can vary considerably from patient to patient and even within a given patient because of the complex metabolism of the drug. In addition, there are several drugs that interact with cyclosporine metabolism, both increasing and decreasing its metabolism (70). Recently, cyclosporine serum level monitoring done 2 hours post-dose has shown better correlation to the drug exposure, reduced renal dysfunction, and no increase in rejection rate (71). The major toxicities of cyclosporine are the induction or exacerbation of hypertension and renal impairment, both of which occur in the majority of patients taking the drug (72). Other less-common side effects include hepatotoxicity, neurotoxicity, seizures, gingival hyperplasia, hirsutism, and hyperglycemia. Most side effects are dose-related and will not preclude its use.
Tacrolimus
This agent was first isolated from Streptomyces tukubaensis in 1994 in Japan. The mechanism of action is quite similar to that of cyclosporine but it has a potency approximately 100 times that of cyclosporine. The initial experience with this agent was in liver transplantation, and over the ensuing years it has become the agent of choice in this setting. More recently, tacrolimus has been used in cardiac transplantation and early reports suggest an equivalent efficacy overall (73).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree