Chapter 29
Cardiac Transplantation
1. How many heart transplantations are performed in the U.S. each year? What are the most frequent causes of heart disease requiring cardiac transplantation?
2. List common indications for heart transplantation.
 Severe heart failure (New York Heart Association [NYHA] class III or IV) with poor short-term prognosis despite maximal medical therapy, requiring continuous inotropic therapy, requiring mechanical support (e.g., balloon pump, left ventricular assist device [LVAD], extracorporeal membrane oxygenation [ECMO])
Severe heart failure (New York Heart Association [NYHA] class III or IV) with poor short-term prognosis despite maximal medical therapy, requiring continuous inotropic therapy, requiring mechanical support (e.g., balloon pump, left ventricular assist device [LVAD], extracorporeal membrane oxygenation [ECMO])
 Restrictive or hypertrophic cardiomyopathy with NYHA class III or IV symptoms
Restrictive or hypertrophic cardiomyopathy with NYHA class III or IV symptoms
 Refractory angina despite medical therapy, not amenable to revascularization, with poor short-term prognosis
Refractory angina despite medical therapy, not amenable to revascularization, with poor short-term prognosis
 Recurrent or refractory ventricular arrhythmias, despite medical and/or device therapy
Recurrent or refractory ventricular arrhythmias, despite medical and/or device therapy
 Complex congenital heart disease with progressive ventricular failure not amenable to surgical or percutaneous repair
Complex congenital heart disease with progressive ventricular failure not amenable to surgical or percutaneous repair
 Unresectable low-grade tumors confined to the myocardium, without evidence of metastasis
Unresectable low-grade tumors confined to the myocardium, without evidence of metastasis
3. What baseline evaluations are obtained in the pretransplantation workup?
Pretransplantation evaluation serves the purpose of assessing a patient’s severity of heart failure, mortality benefit from surgery, comorbidities, and potential contraindications to surgery. Factors important in transplantation evaluation are given in Box 29-1.
4. What are contraindications to heart transplantation?
Contraindications include any noncardiac conditions that may decrease a patient’s survival, and increase risk of rejection or infection, and are listed in Box 29-2.
5. Define allotransplantation versus xenotransplantation, and orthotopic versus heterotopic transplantation.
Allotransplantation involves transplantation of cells, tissue, or organs between same species Xenotransplantation involves transplantation of cells, tissue, or organs between different species. During orthotopic heart transplantation, the donor heart is transplanted in place of the recipient’s heart. There are two anastomotic approaches used (Fig. 29-1).
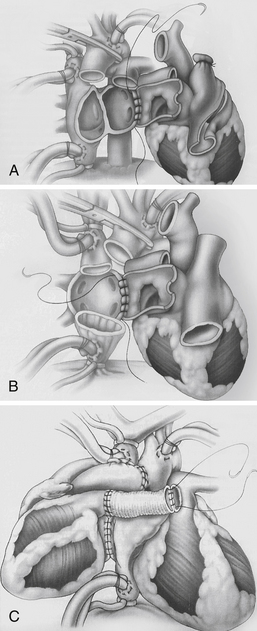
Figure 29-1 Anastomotic approaches used in cardiac transplantation. A, Biatrial approach. The donor atrial cuff is anastomosed to the recipient left atrium, right atrium, followed by aortic and pulmonary artery anastomosis. B, Bicaval approach. The donor left atrial cuff is anastomosed to the recipient left atrium, followed by inferior vena cava, superior vena cava, aortic, and pulmonary artery anastomosis. This approach may be associated with improved atrial function, lower incidence of atrial arrhythmias, sinus node dysfunction, and tricuspid insufficiency. C, Heterotopic transplantation. The donor-recipient left atrial cuff is anastomosed, followed by superior vena cava, aortic, and pulmonary artery anastomosis via Dacron graft. This procedure is considered in donor-recipient body-size mismatch, when the recipient pulmonary artery systolic pressure is greater than 60 mm Hg, or when there is suboptimal donor heart systolic function. (From Kirklin JK, Young JB, McGiffin DC: Heart transplantation, Philadelphia, 2002, Churchill Livingstone.)
 Biatrial approach: The donor atrial cuff is anastomosed to the recipient left atrium, right atrium, followed by aortic and pulmonary artery anastomosis.
Biatrial approach: The donor atrial cuff is anastomosed to the recipient left atrium, right atrium, followed by aortic and pulmonary artery anastomosis.
 Bicaval approach: The donor left atrial cuff is anastomosed to the recipient left atrium, followed by inferior vena cava, superior vena cava, aortic, and pulmonary artery anastomosis. This approach may be associated with improved atrial function, lower incidence of atrial arrhythmias, sinus node dysfunction, and tricuspid insufficiency.
Bicaval approach: The donor left atrial cuff is anastomosed to the recipient left atrium, followed by inferior vena cava, superior vena cava, aortic, and pulmonary artery anastomosis. This approach may be associated with improved atrial function, lower incidence of atrial arrhythmias, sinus node dysfunction, and tricuspid insufficiency.
During heterotopic heart transplantation, the recipient’s heart is left in the mediastinum, and the donor heart is attached “parallel” to the recipient heart (see Fig. 29-1).
6. Define ischemic time of the donor heart. Why is it important?
The cold ischemic time is the time interval between removal of the donor heart and the implantation in the recipient. During this interval, ischemic injury can occur to the heart due to lack of perfusion. Myocardial preservation is achieved with hypothermia and placement of the heart in a solution mimicking intracellular milieu to prevent cellular edema and/or acidosis, and maintain ATP supply for membrane function. A prolonged ischemic time can lead to irreversible damage to the harvested organ. A cold ischemic time of more than 5 hours is associated with a higher incidence of cardiac allograft dysfunction and decreased transplant recipient survival.
7. What is the estimated graft survival at 1 year, 3 years, 5 years, and 10 years posttransplantation? What are the common causes of death?
The major causes of death posttransplantation are as follows:
 Less than 30 days: graft failure, multiorgan failure, infection
Less than 30 days: graft failure, multiorgan failure, infection
 Less than 1 year: infection, graft failure, acute allograft rejection
Less than 1 year: infection, graft failure, acute allograft rejection
 More than 5 years: allograft vasculopathy, late graft failure, malignancies, infection
More than 5 years: allograft vasculopathy, late graft failure, malignancies, infection
8. What is cardiac allograft vasculopathy (CAV)? Describe its pathophysiology, incidence, risk factors, and outcome.
Also known as transplant vasculopathy or transplant coronary artery disease (CAD), CAV is the progressive narrowing of the coronary arteries of the transplanted heart. Angiographic incidence of CAV is approximately 30% at 5 years and 50% at 10 years. CAV is associated with a significantly increased risk of death. After the first year posttransplantation, CAV is the second most common cause of death (after malignancy). In CAV, there is diffuse, concentric proliferation of the intimal smooth muscle cells and it typically involves the entire length of the coronary artery. In contrast, conventional atherosclerosis results from fibrofatty plaque resulting in concentric or eccentric focal lesions. The etiology of CAV remains unclear, but both immunologic (cellular and/or humoral rejection, human leukocyte antigen [HLA] mismatch) and nonimmunologic (cytomegalovirus [CMV] infection, hypercholesterolemia, older age and/or male donors, younger recipients, history of CAD, diabetes mellitus [DM], and insulin resistance) factors have been implicated
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree












































