Fig. 10.1
12-lead ECG in atrial fibrillation with biventricular pacing
Although the patient’s heart failure symptoms improved from NYHA classes III to II and biventricular pacing achieved a success rate of 95 %, multiple episodes of high heart rate AF were detected by the device one month after CRT-D implantation, and oral amiodarone therapy consisting of 200 mg once daily for 5 days per week was started. Three months after the initiation of amiodarone therapy in September 2014, the patient’s heart failure symptoms worsened and blood samples showed an elevated plasma brain natriuretic peptide (BNP). His ECG revealed atrial fibrillation with spontaneous QRS (Fig. 10.2).
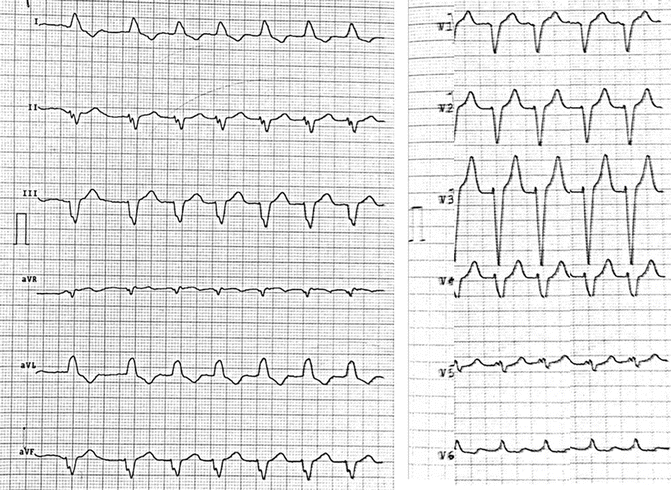

Fig. 10.2
12-lead ECG in atrial fibrillation with spontaneous QRS
Since his heart failure symptoms were refractory to medical management, repeated echocardiography was performed that revealed an increased left atrial diameter to 58 mm and a volume of 46 mL/m2 despite mild improvement of the LVEF. The hypothesis of the worsening clinical condition was that the exacerbation of HF was due to high-rate AF or to displacement of left ventricular (LV) lead; therefore, a chest X-ray was performed and the imaging confirmed the LV lead displacement (Fig. 10.3a, b). Figure 10.4a, b represents the coronary sinus angiography during the reimplantation of new LV pacing lead. Two months after the repositioning of biventricular pacing, echocardiography demonstrated an increase in the LVEF from 28 to 38 % and a reduction in the left atrial diameter from 58 to 56 mm and the left atrial volume from 46 to 45 mL/m2 compared with the values examined in December 2014.
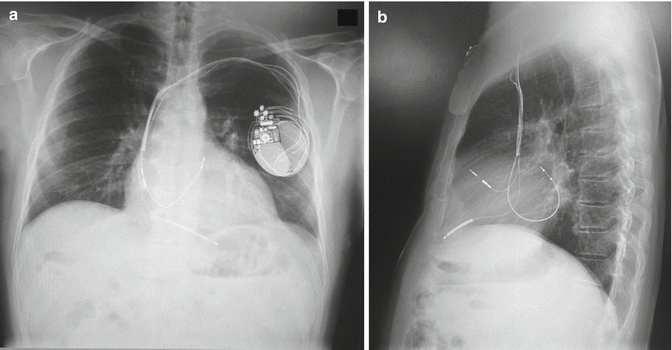
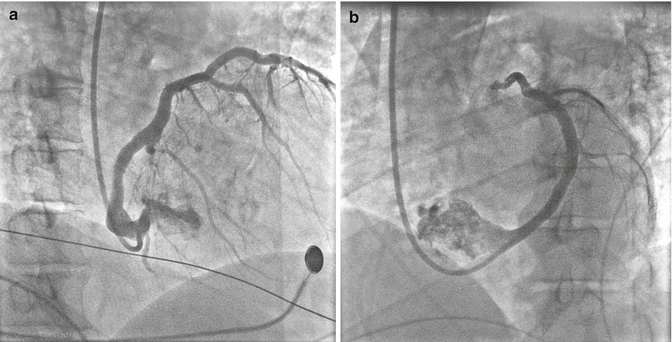

Fig. 10.3
(a) Posteroanterior chest X-ray showing the left ventricular lead displacement. (b) Latero-lateral chest X-ray confirming the left ventricular lead displacement

Fig. 10.4
(a) Coronary sinus angiography on right anterior oblique projection. (b) Coronary sinus angiography on left anterior oblique projection
The patient’s clinical course was satisfactory, and his plasma BNP level decreased to 504 pg/mL 2 months after the repositioning of the LV lead.
10.2 Cardiac Resynchronization Therapy (CRT)
Definition
Cardiac resynchronization therapy (CRT) is a specific type of pacemaker therapy that aims to restore or improve ventricular contraction by pacing both the right ventricle (RV) and the left ventricle (LV). Indeed, in addition to the right lead, an extra catheter is usually implanted via the coronary sinus to pace the LV, most commonly at the latest activated LV segment. This may be carried out with or without the use of an implantable cardioverter defibrillator (ICD), a device required in patients at risk for ventricular arrhythmias [1].
Left bundle branch block (LBBB) is an electrocardiographic sign of electrical dyssynchrony and, in association with poor LV function, is an independent risk factor for mortality [2]. LBBB often correlates with mechanical dyssynchrony also called interventricular delay, a nonphysiological timing of LV and RV contraction. There may also be abnormal contraction of individual segments of the LV, causing intraventricular delay. In an HF patient, the inter- and intraventricular dyssynchrony can further worsen the pump function of a failing LV. CRT, through the reestablishment of a more physiological ventricular activation, improves cardiac performance and reduces HF symptoms and also determines a reverse remodeling of the LV along with mortality and hospitalization reduction [3–7].
CRT is usually set in DDD or DDDR mode if the patient is in sinus rhythm. Atrial events trigger an atrioventricular interval that should be long enough in order to allow the optimal atrial contribution to ventricular filling but short enough to allow ventricular pacing for most of the time. As specified in the ESC guidelines [1], the goal of CRT should be to achieve BiV pacing as close to 100 % as possible since the reduction in mortality and hospitalization is strongly associated with an increasing percentage of BiV pacing. In addition, CRT pacing often results in a QRS complex that is narrower than the native QRS complex because of fusion between the two paced signals.
Clinical Trials
CRT has been clinically evaluated in more than 4000 patients in randomized controlled trials (Table 10.1). Early studies were positive in terms of morbidity, including improvements in quality of life, functional status, and exercise capacity. Finally, the large trial COMPANION [3], along with the CARE-HF trial [4], provided the demonstration of mortality benefit in HF patients, defined as LVEF <35 %, QRS duration ≥120 ms, and NYHA functional class III or ambulatory class IV HF. Recently, three major randomized trials [MADIT-CRT [5], RAFT [6], and REVERSE [7]] have demonstrated that CRT can provide functional improvement and decrease the heart failure events in patients with reduced LVEF and NYHA class I or II HF and QRS ≥120 or 130 ms. CRT-D has also been shown to decrease the mortality rate for patients with class II but not class I HF. Further subgroup analyses of data from MADIT-CRT, REVERSE, and RAFT trials demonstrated that a CRT implant in patients with a QRS duration ≥150 ms yielded a greater survival benefit compared to a QRS duration of 120 ms. Also meta-analyses using aggregate data from randomized trials confirmed that CRT was effective in reducing adverse clinical events in patients with baseline QRS duration ≥150 ms and proposed that CRT might not reduce events in patients with a QRS <150 ms [8].
Table 10.1
Summary of major studies of CRT
Trials | Patients, no. | NYHA class | LVEF, % | QRS, ms | Primary end points | Secondary end points | Main findings |
|---|---|---|---|---|---|---|---|
COMPANION [3] | 1520 | III or IV | 21 | 159 | (1) All-cause mortality or hospitalization | (2) All-cause mortality and cardiac mortality | CRT-P/CRT-D reduced (1) and (2) |
CARE-HF [4] | 813 | III or IV | 25 | 160 | (1) All-cause mortality or hospitalization | (2) All-cause mortality | CRT-P reduced (1) and (2) |
REVERSE [7] | 610 | I or II | 27 ± 7 | 153 | (1) HF clinical composite score | (2) LVESVi, (3) HF hospitalization, (4) all-cause mortality < div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|