Cardiac Resynchronization Therapy (CRT)
How it Works
Cardiac resynchronization therapy (CRT), also referred to as biventricular pacing, is aimed at improving the disordered patterns of ventricular contraction—referred to as ventricular dyssynchrony—seen in some patients with heart failure. CRT is accomplished, in general, by pacing both ventricles simultaneously, thus improving the coordination of dyssynchronous left ventricular contraction.
CRT pacemakers have three pacing leads instead of two: a right atrial lead, a right ventricular lead, and a left ventricular lead. They work similarly to DDD pacemakers except for two things. First, with CRT pacing, both ventricles are paced instead of just the right ventricle. Second, biventricular pacing itself, rather than rate support, is the primary desired therapy—CRT pacemakers are thus programmed to pace virtually 100% of the time, under all conditions.
CRT pacemakers are used to correct ventricular dyssynchrony. Ventricular dyssynchrony is most obviously present in patients with wide QRS complexes. Since the QRS complex reflects the activation sequence of the ventricular muscle, a wide QRS complex indicates that the ventricles are being activated abnormally. Specifically, a bundle branch block implies that one ventricle is being activated before the other—that is, the ventricles are being activated sequentially instead of simultaneously.
This sort of ventricular dyssynchrony does not produce any noticeable hemodynamic consequences in people with otherwise normal hearts. But in patients with systolic dysfunction, ventricular dyssynchrony can produce enough inefficiency in ventricular contraction to cause or worsen symptoms of heart failure and, potentially, can exacerbate ventricular remodeling and a reduction in the ventricular ejection fraction. In these cases, by pacing both ventricles simultaneously one can often resynchronize ventricular contraction sufficiently to improve ventricular efficiency, reduce symptoms, and improve clinical outcomes.
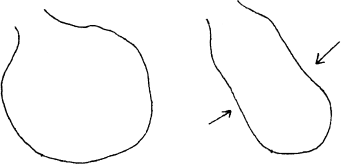
Figure 9.1 illustrates how left ventricular dyssynchrony caused by left bundle branch block (LBBB) can affect ventricular function in a patient with heart failure. The panel on the left shows a dilated left ventricle at end-diastole. The middle panel shows the first 60 msec of systole. Here, the septum (which is activated along with the right ventricle) is already contracting, but the left ventricular free wall has not yet been activated (due to the bundle branch block). In fact, the left free wall bulges outward. The panel on the right shows the last 60 msec of systole. The left ventricular free wall has finally been activated and is contracting, but now the septum, which has already finished contracting, is pushed outward. As a result, with each systole, much of the precious energy being expended by this diseased ventricle is used to create a useless, swaying, “hula”-type movement, swishing blood around inside the cardiac chamber instead of ejecting it out into the aorta.
Figure 9.1 How ventricular dyssynchrony causes reduced ventricular function. The three panels in this figure illustrate ventricular systole in a patient with cardiomyopathy and LBBB. The panel on the left shows the dilated left ventricle at end-diastole. The middle panel shows the first 60 msec of systole. Here, the septum (which is activated along with the right ventricle) is already contracting, but the left ventricular free wall has not yet been activated. In fact, the free wall bulges outward. The panel on the right shows the last 60 msec of systole. The left ventricular free wall is now finally being activated, but now the septum, which has already finished contracting, is pushed outward. As a result of this dyssynchrony, with each systole much of the energy expended by this diseased ventricle is applied toward creating a useless, swaying, “hula”-type movement, instead of toward ejecting blood into the aorta.
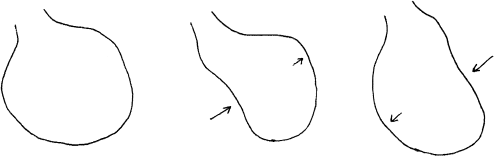
Figure 9.2 illustrates how CRT might benefit such a ventricle. The panel on the left again shows the same dilated left ventricle at end-diastole. The panel on the right shows what happens when biventricular pacing is activated. Here, the right and left ventricles are paced simultaneously; both the septum and the left ventricular free wall contract at the same time. The energy expended by the ventricle now goes toward ejecting blood. Ventricular contraction becomes much more efficient and effective.
Figure 9.2 How biventricular pacing improves ventricular function. The two panels in this figure illustrate how biventricular pacing improves the function of the dyssynchronous, cardiomyopathic left ventricle illustrated in Figure 9.1. The panel on the left shows the dilated left ventricle at end-diastole. The panel on the right shows what happens when biventricular pacing is activated. Here, the right and left ventricles are paced simultaneously; both the septum and the left ventricular free wall contract at the same time. The energy expended by the ventricle now goes toward ejecting the blood, instead of merely swishing it around inside the cardiac chamber. Ventricular contraction becomes much more efficient and effective.
Between 20 and 30% of patients with congestive heart failure have intraventricular conduction delays, and in most of these it is left ventricular activation that is delayed.
The Effects of CRT
Several clinical studies have documented the benefits of CRT in appropriately selected patients.
Hemodynamic Effects
CRT has consistently yielded improved hemodynamic function in patients with heart failure and LBBB, including improved cardiac output and cardiac index, increased aortic pulse pressure, and reduced pulmonary capillary wedge pressure.
Contractility
Measures of left ventricular contractility improve with CRT, including enhanced global contraction and increased left ventricular ejection fractions. In contrast to other forms of therapy that have boosted ventricular contractility in patients with heart failure (such as the inotropic agents amrinone and milrinone), CRT actually reduces myocardial energy expenditures. Thus, ventricular contraction is not only more effective but also more efficient.
Reverse Remodeling
Remodeling of the left ventricle—manifested by ventricular dilation, increased ventricular mass, and reduced ejection fraction—is a fundamental response to reduced systolic function. In essence, the cardiac enlargement that occurs with remodeling is a compensatory mechanism which allows the ventricle to eject a near-normal stroke volume despite reduced contractility. Aside from the fact that remodeling itself is ultimately harmful, the degree of remodeling reflects the degree of systolic dysfunction.
CRT has been demonstrated to reverse left ventricular remodeling. Specifically, it has been shown to reduce the end-systolic and end-diastolic dimensions of the left ventricle, as well as the left ventricular mass. This reverse remodeling is thought to reflect a fundamental improvement in ventricular systolic function.
Clinical Studies with CRT
Numerous clinical trials have now been completed to assess the benefits of CRT in patients with heart failure due to systolic dysfunction. The results of these trials can be summarized as follows.
Patients with Moderate to Severe Heart Failure
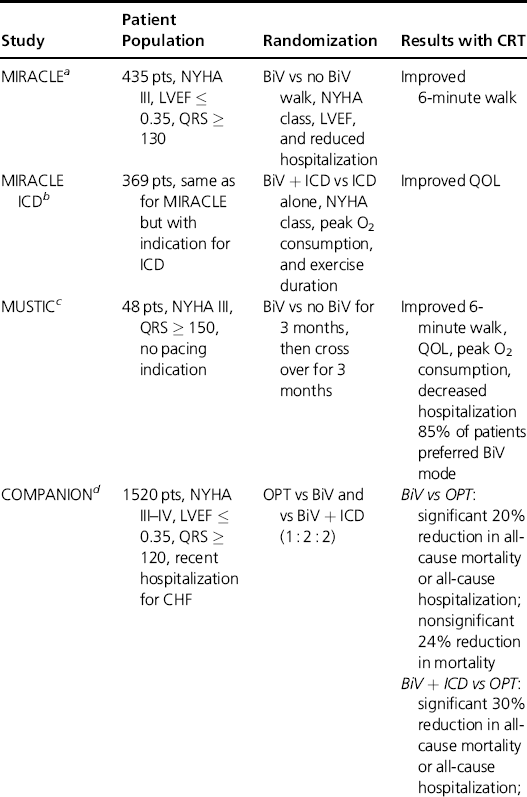
The earliest clinical trials with CRT generally enrolled patients who had significant heart failure symptoms—generally, patients with NYHA class III or IV heart failure. In addition, these patients had QRS prolongation (at least 120–140 msec) and left ventricular ejection fractions of 0.35 or less. The major clinical trials in such patients are summarized in Table 9.1. In general, these trials strongly suggest that CRT in appropriately selected patients with severe heart failure can significantly reduce heart-failure mortality and hospitalizations, improve left ventricular ejection fractions, and improve quality of life.
Table 9.1 Major randomized trials with CRT in patients with moderate to severe heart failure due to systolic dysfunction and wide QRS complexes. These trials confirmed the effectiveness of CRT in improving functional capacity, quality of life, need for hospitalization, and survival. From the COMPANION trial, the addition of an ICD to CRT therapy greatly improves on the survival benefit seen with CRT alone.

In addition, a meta-analysis of 14 controlled, randomized trials that included over 1400 patients with moderate to severe heart failure has been published (McAlister FA et al., JAMA 2007; 297:2502). This meta-analysis suggests that patients receiving CRT, when compared to optimal medical therapy, have a much higher chance of improving at least one NYHA class (59 vs 37%), a heart-failure hospitalization rate that is reduced by more than 35%, improved exercise capacity and quality-of-life measures, and reduced heart-failure mortality and overall mortality.
Patients with Mild to Moderate Heart Failure
Two major randomized clinical trials have now been published evaluating the effects of CRT in patients with systolic dysfunction and intraventricular conduction delays but who have only NYHA class I or II heart-failure symptoms.
The REVERSE trial (Linde C et al., J Am Coll Cardiol 2008; 52:1834) enrolled 610 patients with NYHA class I or II heart failure, QRS duration ≥120 msec, left ventricular ejection fraction <40%, and left ventricular end-diastolic diameters ≥55 mm. The patients all received CRT devices, but they were randomized as to who had biventricular pacing turned on or off. After 12 months, patients randomized to CRT had reduced hospitalizations and improved ejection fractions, but the proportion of patients who clinically worsened was not reduced by CRT pacing.
However, the 262 REVERSE patients who had been enrolled in Europe remained in their randomized pacing modes for 24 months, rather than 12. Among this subset of patients, clinical worsening was significantly reduced in those assigned to CRT pacing.
The MADIT-CRT trial (Moss AJ et al., N Engl J Med 2009; 361:1329) compared ICD therapy to ICD combined with CRT pacing in 1820 NYHA class I or II patients who had reduced left ventricular ejection fractions (30% or lower) and QRS durations of at least 130 msec. After a follow-up averaging 29 months, those who received CRT pacing had a significant reduction in the primary endpoint, which was death from any cause or a nonfatal heart failure event—though the main benefit appears to have been a reduction in heart-failure episodes. In this trial, patients with LBBB and QRS duration >150 msec received the most benefit from CRT.
Indications for CRT
In general, CRT is indicated in patients who have heart failure from systolic dysfunction and significant ventricular dyssynchrony. Guidelines from medical societies to this point have defined “dyssyncrhony” solely by QRS duration, and different sets of guidelines have used different cutoffs for QRS duration.
Guidelines published in 2008 by the American College of Cardiology, American Heart Association, and Heart Rhythm Society (Epstein AE et al., Circulation 2008; 117:e350) give the major indications for CRT as follows:
- CRT is recommended in patients in NYHA class III or IV, despite optimal medical therapy, who have left ventricular ejection fractions of 35% or less, and QRS durations of 120 msec or more.
- CRT is reasonable in patients in NYHA class III or IV, despite optimal medical therapy, who have left ventricular ejection fractions of 35% or less, and are largely dependent on ventricular pacing—whatever their native QRS duration might be.
- CRT can be considered in patients in NYHA class I or II, on optimal medical therapy, who have left ventricular ejection fractions of 35% or lower, who will be receiving a permanent pacemaker or ICD, and who are expected to require frequent ventricular pacing.
Guidelines published by medical societies have not yet taken into account the results of REVERSE and MADIT-CRT. However, considering the results of these trials, it seems reasonable to implant defibrillators that provide CRT pacing (referred to as CRT-D devices) in patients with NYHA class I or II heart failure, left ventricular ejection fractions of 30% or less, and QRS durations of 150 msec or more.
In fact, it ought to be noted that the vast majority of patients with an indication for CRT therapy will also have an indication for an implantable defibrillator; so the great majority of patients with heart failure who are candidates for CRT should receive CRT-D devices.
Implanting CRT Devices
The only significant difference between implanting a CRT device and a pacemaker (or between implanting a CRT-D device and an ICD) is the need to place an additional lead for left ventricular pacing.
In the early days of CRT, left ventricular pacing was accomplished either with epicardial leads (requiring a limited thoracotomy) or with standard transvenous pacing leads—leads not designed for this purpose—placed into the coronary sinus. Left ventricular lead placement, in those days, was a lengthy, tedious, difficult, and often risky procedure. Nowadays, a variety of tools have been developed specifically for placement of specially designed pacing leads in the coronary venous system. These tools allow the electrophysiologist to rapidly and safely insert left ventricular pacing leads via the coronary sinus. In most cases, these leads can be placed, positioned, and tested in 30 minutes or less.
In placing left-sided leads, the os of the coronary sinus is generally first engaged with an introducer designed specifically for this purpose. Dye is injected to visualize the cardiac venous system. A “target” vein is identified. (Most patients seem to do best with CRT when the left-sided lead is placed in the mid-lateral left ventricle, so the operator looks for a vein that reaches that area.) A pacing lead is chosen whose handling characteristics are likely to suit the anatomy of the target vein, and is then inserted and positioned.
In testing the left-sided lead, the operator looks not only for adequate R wave voltage, pacing threshold, and impedance measurements, but also for evidence of diaphragmatic stimulation—the most common problem with pacing from the coronary veins. If diaphragmatic stimulation is seen, the lead needs to be repositioned. Coronary sinus leads are now available that can be “electronically repositioned” by changing the vector configurations of available electrodes. Diaphragmatic stimulation can usually be circumvented in this way without having to physically reposition the lead.
Coronary sinus perforation and subsequent pericardial tamponade is the most feared procedural complication unique to left-sided lead placement. Additional complications with CRT implantation include diaphragmatic pacing, pneumothorax, and infection.
Adequate placement of a left ventricular lead can be accomplished today via the coronary sinus approach in well over 90% of patients. Occasionally, however, pacing the left ventricle still requires an epicardial lead. Tools are being developed to make epicardial lead placement much less invasive and more reliable than it is today; it is likely that within several years, placing epicardial leads will be conducted routinely in the electrophysiology laboratory.
Unresolved Issues with CRT
Responders Versus Nonresponders
From the very earliest days of CRT therapy, doctors took note of the fact that many patients with heart failure who received these devices had very dramatic responses. These patients improved rapidly from NYHA class III to I, or from class IV to II, and were able to accomplish physical tasks they had not been able to perform for months or years. These patients were exceedingly grateful for their new lease on life and, accordingly, the doctors were exceedingly gratified. Such patients were quickly deemed to be “responders.” Roughly 40–60% of patients who receive indicated CRT devices for heart failure fall into this category.
Naturally, patients who did not have such dramatic improvements in well-being began to be regarded as “nonresponders.” This designation is probably unfortunate.
The tendency to consider the lack of a dramatic response to CRT as equivalent to a lack of any meaningful response is shortsighted. It seems very unlikely that CRT will produce an “all-or-nothing” effect, where either patients have remarkable, raising-the-dead-style symptomatic improvement or no improvement at all. More likely, some patients are benefited by CRT in a more subtle way, such that, while they may not feel dramatically better, the “trajectory” of their illness improves, so that they have fewer hospitalizations over a given period of time or an improvement in mortality.
As it turns out, these more subtle benefits of CRT are the very benefits that the randomized trials (discussed earlier) were designed to measure. From these trials, the magnitude of overall benefit to the population probably cannot be explained by the 40–60% of patients who (if the “responder”/“nonresponder” parameter had been tabulated) would have been classified as “dramatic responders”. For instance, in the CARE-HF study, mortality in the CRT group was reduced by 33%, a magnitude that would be very difficult to attribute to the 50% or so who likely responded “dramatically” to the therapy. More likely, this impressive benefit in CARE-HF was distributed among both “dramatic responders” and “nondramatic responders.”
And finally, evidence is accumulating that the more subtle measures of CRT efficacy—such as reverse remodeling—may continue to improve for at least 12 months after CRT is initiated, even in so-called “nonresponders.” This reverse remodeling suggests that some fundamental improvements in ventricular systolic function are occurring over time, and may be present whether or not a dramatic reduction in symptoms has been seen.
If we clinicians follow our understandable tendency to define “responders” by the presence or absence of a dramatic response to CRT, we will create unreasonable expectations on the parts of patients, payers, and ourselves. If we must identify certain individuals as nonresponders, we at least ought to be circumspect about how we do so. We should define “nonresponder” in a way that allows for the more subtle but still substantial benefits of CRT. Doing so will improve our chances of actually figuring out how to maximize the benefits of CRT. It will also reduce the temptation we otherwise create for payers to withhold CRT from anyone who doesn’t seem likely to turn cartwheels within 48 hours of implantation.
Optimization of CRT
Beyond the original notion that pacing the ventricles simultaneously will help to resynchronize dyssynchronous ventricular contraction, relatively little has been accomplished so far in systematically studying how the benefits of CRT might be optimized in each individual patient. Several methods for optimizing CRT have been proposed, and, if developed sufficiently, one or more of these might improve the overall benefits of CRT.
AV Interval
Despite the spotted history (described earlier) of AV-interval optimization in improving the hemodynamics of failing hearts, there is reason to believe that AV optimization may be an important factor in CRT.
Specifically, the chief concern is with the left atrial to left ventricular (LA–LV) interval. Since pacemakers—even CRT pacemakers—sense only the right (and not the left) atrial electrogram, any intraatrial conduction delay (i.e. a conduction delay from the right to the left atrium) is not taken into account. In other words, the “A” in “AV interval” comes from the right atrium—the left atrium is ignored. Therefore, in the setting of an intraatrial conduction delay, during CRT the programmed AV delay may result in an effective LA–LV interval that is “too short,” such that left ventricular pacing may occur before left atrial contraction is completed. As a result, left ventricular stroke volume may be systematically reduced during CRT.
Whether this is more than a theoretical problem, and whether optimization of LA–LV intervals would improve the effects of CRT in some or all patients, has not been sufficiently studied. It is certainly one area of concern in the optimization of CRT.
VV Interval
Classically, CRT is accomplished by pacing the left ventricle simultaneously with right ventricular activation (or pacing). It may, however, be the case that different timing sequences between the two ventricles (the VV interval) would improve the efficacy of CRT in some patients. Data exist, for instance, suggesting that in some patients, pacing only the left ventricle (in advance of any right ventricular activation) might be better than biventricular pacing.
The VV interval ought to be viewed as a continuum of potential ventricular activation sequences, all the way from right-ventricular-only pacing (i.e. the pacing mode used in all “standard” pacemakers) to left-ventricular-only pacing. For all we know, the optimal VV interval may vary from patient to patient.
CRT devices exist today that allow the physician to vary the VV interval—but this feature is marketed with few instructions. The clinician has no objective guidance on how to choose the most appropriate VV interval. Systematic studies are needed to assess whether varying the VV intervals makes a substantial difference, and, if so, how exactly to optimize this parameter in individual patients.
Lead Placement and Lead Number
When one stops to think about it, it begins to seem remarkable that we have seen such impressive results with CRT when positioning the left ventricular pacing leads “empirically,” often in whichever location is most readily achievable.
Could the results of CRT be improved by objectively assessing which specific region within the left ventricle ought to be paced in each individual, and then taking pains to position the lead in the optimal spot? Some evidence exists (from MRI studies) suggesting that this may be so. Specifically, there may exist in at least some dyssynchronous left ventricles a “sweet spot”—generally the area of the left ventricle with the most delayed activation. Placing the pacing electrode in that spot might yield much better resynchronization than placing it just a few centimeters away. Perhaps some patients have more than one “sweet spot,” and perhaps two or more left ventricular electrodes would need to be positioned, quite precisely, in order to truly optimize CRT. Optimal lead placement—and the optimal number of leads to use—is another area in need of systematic investigation.
Measuring Dyssynchrony
The methodology we currently use to detect left ventricular dyssynchrony—looking for the presence or absence of an intraventricular conduction delay—is neither particularly sensitive nor specific. Substantial ventricular dyssynchrony can exist in the absence of an obvious conduction delay, and some patients with conduction delays may not have much dyssynchrony at all.
If CRT truly works by resynchronizing the contraction of a dyssynchronous left ventricle, then it seems obvious that to really optimize CRT we need to have an objective, reproducible way to measure dyssynchrony, both before and during CRT pacing. Unfortunately, we do not. Cardiac MRI shows much promise in this area but is not readily available—and it is problematic to perform MRI scans in patients with CRT devices. Tissue Doppler echocardiography (TDE) is also promising, but remains poorly standardized, and its results sometimes appear to be quite operator-dependent. Accordingly, in 2008 (Gorcsan J 3rd et al., J Am Soc Echocardiogr 2008; 21:191) the American Society of Echocardiography (in what has to have been a painful process) released a consensus statement urging that patients who otherwise have an indication for CRT should not have CRT therapy withheld because of the results of an echocardiographic dyssynchrony study; and further, that echocardiography reports should not include a recommendation regarding whether CRT is likely to be beneficial or not.
While current methods fall short of the goal, the clinical need for a method of reproducibly measuring ventricular dyssynchrony has now been widely recognized. A lot of work currently aims at accomplishing this, and it is probably only a matter of time before this need is met. When that happens, we can expect improved tools for fully optimizing CRT to follow rapidly.
Should CRT be the Standard Mode of Pacing?
If spontaneous LBBB produces ventricular dyssynchrony, and is thus detrimental to patients with systolic heart failure, then wouldn’t iatrogenic LBBB created by right ventricular pacing also be detrimental to these patients? Is it really a good idea for patients who require pacing most or all of the time to have LBBB-inducing right ventricular pacing—especially patients with systolic dysfunction?
At least three trials suggest that chronic right ventricular pacing is detrimental in patients with systolic dysfunction. In the DAVID trial (Wilkoff BL et al., JAMA 2002; 288:3115), patients who needed ICDs and who also had depressed left ventricular function were randomized to DDDR pacing with a lower rate limit of 70 beats/min (in order to increase the use of dual-chambered pacing) or to VVI pacing at a lower rate of 40 beats/min (to decrease the use of any pacing at all). The hypothesis of this study—which was initiated before CRT and its implications regarding ventricular dyssynchrony were widely known—was that chronic AV pacing would be beneficial in these patients. Instead, the investigators discovered the opposite. Patients randomized to frequent DDDR pacing had a significantly higher incidence of death or hospitalization for heart failure than patients randomized to minimal pacing. Most observers attribute these negative results to the dyssynchrony produced by almost constantly pacing the right ventricle in patients with underlying left ventricular dysfunction.
Similarly, the MOST trial (Sweeney MO et al., Circulation 2003; 107:2932) examined clinical outcomes, using various pacing modes, in patients with sinus nodal dysfunction. In this trial, patients with higher cumulative percentages of right ventricular pacing also had more hospitalizations for heart failure.
Most telling of all is the PAVE trial (Doshi RN et al., J Cardiovasc Electrophysiol 2005; 16:1160), in which 184 patients with atrial fibrillation and NYHA class II or III heart failure who received AV node ablation for rate control were randomized to standard RV pacing or to CRT pacing. Those who received CRT pacing had better 6-minute walking duration, better peak O2 consumption during exercise, better exercise duration, and better preservation of left ventricular ejection fractions than those receiving standard right ventricular pacing. Subsequently, the US Food and Drug Administration approved CRT pacing for patients with NYHA class II or III heart failure and atrial fibrillation who undergo AV node ablation for rate control.
So what we have learned in the last few years is that CRT pacing is almost certainly superior to right ventricular pacing in patients with moderate heart failure who are likely to be in a paced rhythm all the time.
Whether CRT pacing would also yield more favorable long-term results even in patients who did not have systolic dysfunction is an intriguing question. In the PACE trial (Yu CM et al., N Engl J Med 2009; 361:2123), 177 patients with normal ventricular function were randomized to RV pacing versus CRT pacing. At 12 months, those receiving CRT had significantly higher left ventricular ejection fractions and lower left ventricular end-systolic volumes than those receiving RV pacing.
However, in order for CRT pacemakers to be used routinely in patients who require pacing, we will need not only more clinical evidence to prove that CRT is significantly better than RV pacing in nearly all pacemaker-dependent patients, but also improvements in the ease and safety of implanting left-sided leads. Accordingly, several manufacturers of CRT pacemakers are working hard to improve the tools for placing left-sided leads.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


