1
Secondary prevention
Clinical history of ASCVD
2
Primary prevention
LDL-C ≥190 mg/dL, age ≥21 years
3
Primary prevention
Diabetes: age 40–75 years, LDL-C 70–189 mg/dL
4
Primary prevention
No diabetes: ≥7.5 % 10-year ASCVD risk, age 40–75 years, LDL-C 70–189 mg/dL
Optional candidates for statin therapy
≥5 % 10-year ASCVD risk
Family history of premature ASCVD
hs-CRP ≥2.0 mg/L
CAC score ≥300 or ≥75th percentile,
Ankle-brachial index <0.9
LDL-C ≥160 mg/dl
1.
Individuals with a history of clinical ASCVD should receive statin therapy (high-intensity statin for persons ≤75 years and moderate for those >75 years).
2.
Primary prevention individuals ≥21 years with LDL-C ≥190 mg/dL (which is in the range concerning for a genetic dyslipidemia) should receive statin therapy (preferably high intensity), and it is reasonable to achieve at least a 50 % reduction in LDL-C (additional non-statin therapy if needed to achieve this target may be considered).
3.
Primary prevention individuals aged 40–75 years with diabetes and LDL-C ≥70 mg/dL should receive at least moderate statin therapy, and it is reasonable to consider high-intensity statins when ASCVD risk ≥7.5 % over the next decade.
4.
Primary prevention individuals aged 40–75 years without diabetes but who have LDL-C ≥70 mg/dL and elevated ASCVD risk ≥7.5 % should receive moderate- or high-intensity statins after a clinician-patient discussion (moderate statins may also be considered for those with ASCVD risk 5–7.5 % or with elevated novel risk markers as described above). The patient with ASCVD risk >7.5 % who does not have diabetes or clinical evidence of ASCVD should not be given statin therapy without clinician-patient risk discussion. Patient preference needs to be considered in initiation of statin in this group of patients.
High-intensity statins include atorvastatin 40–80 mg and rosuvastatin 20–40 mg and should reduce LDL-C ≥50 %. Moderate-intensity statins should reduce LDL-C 30–50 % and include atorvastatin 10–20 mg, rosuvastatin 5–10 mg, simvastatin 20–40 mg, pravastatin 40–80 mg, lovastatin 40 mg, fluvastatin 40 mg twice daily, and pitavastatin 2–4 mg (Chap. 28). Of note, the guidelines do not recommend statins in persons on hemodialysis or those with advanced heart failure (due to lack of benefit in randomized clinical trials such as AURORA and CORONA).
While LDL-C targets are no longer endorsed by the guidelines, regular monitoring of statin therapy for adherence and safety is recommended. In order to monitor therapeutic response, a fasting lipid panel should be performed within 4–12 weeks of initiation and 3–12 months thereafter. Most clinicians will likely choose to recheck a lipid profile on a yearly basis in stable patients to assess compliance and to look for signs of new metabolic conditions (such as hypothyroidism) that may lead to worsening of the lipid profile. Individuals receiving statins should be monitored for new onset diabetes, especially those who are receiving high-intensity statin therapy and those with preexisting glucose intolerance [10]. While the guidelines do not routinely recommend non-statin therapies, it is reasonable to consider alternatives for persons who are completely statin intolerant or cannot achieve the recommended intensity of statin.
It is important to emphasize that a central tenet of these new cholesterol treatment guidelines is that, while four specific groups of “statin benefit” are endorsed for consideration of statin initiation, the final decision to start therapy should only occur after a full clinician-patient risk discussion (particularly for the fourth, primary prevention, group). This discussion should cover the following: heart-healthy lifestyle, the potential for ASCVD risk reduction, the potential for side effects, management of other risk factors, patient preferences, and whether any further testing may be necessary when the decision is unclear. This discussion is particularly important as there have been concerns for overuse of statin therapy based in these guidelines [8]. Patient preferences regarding lifelong preventive therapies should be given more priority [11]. A summary of the new cholesterol treatment recommendations is provided in Fig. 2.1.
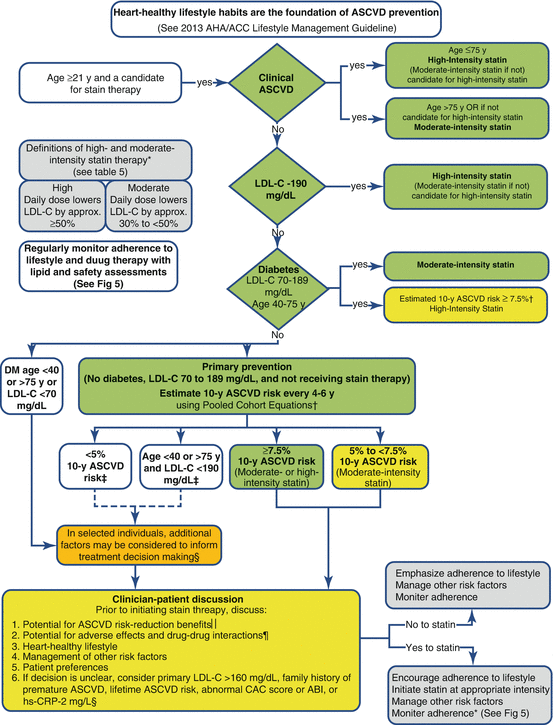

Fig. 2.1
American Heart Association-American College of Cardiology Statin Initiation guidelines for the treatment of blood cholesterol to reduce ASCVD risk in adults. Colors correspond to the classes of recommendation (green = class 1, yellow = class 2a, orange = class 2b). *Percent reduction in LDL-C can be used as an indication of response and adherence to therapy but is not in itself a treatment goal. †The Pooled Cohort Equations can be used to estimate 10-year ASCVD risk in individuals with and without diabetes. The estimator within this application should be used to inform decision making in primary prevention patients not on a statin. ‡Consider moderate-intensity statin as more appropriate in low-risk individuals. §For those in whom a risk assessment is uncertain, consider factors such as primary LDL-C 160 mg/dL or other evidence of genetic hyperlipidemias, family history of premature ASCVD with onset <55 years of age in a first-degree male relative or <65 years of age in a first-degree female relative, hs-CRP 2 mg/L, CAC score 300 Agatston units, or 75th percentile for age, sex, and ethnicity (for additional information, see http://www.mesa-nhlbi.org/CACReference.aspx), ABI <0.9, or lifetime risk of ASCVD. ‖Potential ASCVD risk-reduction benefits. The absolute reduction in ASCVD events from moderate- or high-intensity statin therapy can be approximated by multiplying the estimated 10-year ASCVD risk by the anticipated relative risk reduction from the intensity of statin initiated (approx. 30 % for moderate-intensity statin or approx. 45 % for high-intensity statin therapy). The net ASCVD risk-reduction benefit is estimated from the number of potential ASCVD events prevented with a statin, compared to the number of potential excess adverse effects. ¶Potential adverse effects. The excess risk of diabetes is the main consideration in approx. 0.1 excess cases per 100 individuals treated with a moderate-intensity statin for 1 year and approx. 0.3 excess cases per 100 individuals treated with a high-intensity statin for 1 year. In RCTs, both statin-treated and placebo-treated participants experienced the same rate of muscle symptoms. The actual rate of statin-related muscle symptoms in the clinical population is unclear. Muscle symptoms attributed to statin therapy should be evaluated. ABI indicates ankle-brachial index, ASCVD atherosclerotic cardiovascular disease, CAC coronary artery calcium, hs-CRP high-sensitivity C-reactive protein, LDL-C low-density lipoprotein cholesterol, MI myocardial infarction, RCT randomized controlled trial (Reproduced from open source reference [1]
While there is no doubt that these cholesterol treatment guidelines represent a major step forward in preventive cardiology, there have been some criticisms (Table 2.2). Most notably was the concern that statins may become overprescribed in the setting of overestimation of risk using the new ASCVD risk calculator, which is heavily influenced by chronologic age [12]. In addition, there have been concerns about the loss of LDL-C targets for therapy. The authors of the guidelines removed these targets because there was no randomized evidence that treatment to specific LDL-C targets is beneficial (statins in randomized trials were allocated based on intensity and dosage) and because there was concern that recommending specific targets may motivate the use of non-statin medications (which are mostly of unproven efficacy) just to get patients to arbitrary LDL-C levels. However, there is biologic rationale to justify LDL-C targets, particularly in higher-risk secondary prevention [13].
Table 2.2
Pros and cons of the new cholesterol treatment guidelines
Pros | Broadening of risk assessment to include consideration of stroke, with separate risk equations by gender and race |
Identifying statins as the first-line treatment | |
Emphasizing the dose of statin and adherence to statin | |
Education on managing statin side effects | |
Collaboration between different writing groups and use of high-quality evidence from randomized trials | |
Cons | Abandonment of lipid goals without evidence for superiority of the new approach |
Strong reliance on a risk equation that is heavily weighted by chronological age (without consideration of family history or vascular age) and has only modest discriminatory capacity for events | |
Use of additional testing like CAC only to up-classify risk but not to downgrade risk | |
Little explicit integration of CAC and choice of threshold of >300 or >75th percentile rather than Agatston score of >100 | |
Ignores compelling observational evidence that supports LDL-C targets |
Similarly, monitoring of LDL-C may identify persons with high residual risk and also may justify consideration of non-statin medications (particularly in those with atherogenic dyslipidemia/metabolic syndrome). In addition, non-HDL-C targets may better capture the treatment effect of statins and other therapies on triglyceride-rich remnant lipoprotein burden [14]. Further, these guidelines do not provide specific recommendations on the use of non-statin medications – only to recommend considering non-statin therapy in high-risk patients who have not responded to statins appropriately or are not able to tolerate statins. Finally, LDL-C targets remain in European, Canadian, and other national guideline recommendations, representing a current lack of international consensus on this issue [15]. However, despite these concerns, it is widely considered that implementation of these recent guidelines is likely to improve the cardiovascular health of the population overall.
2.5 Lifestyle Management Guidelines
As this textbook focuses on the pharmacotherapy of cardiovascular disease, we will only cover the highlights from guidelines on lifestyle management to reduce ASCVD risk (Chap. 30). Diet was a major focus of these guidelines, and overall, these guidelines emphasize dietary patterns rather than individual dietary components. Other critical questions addressed included the effect of dietary sodium and potassium on ASCVD risk and the effect of physical activity. Based on rigorous systematic review of the evidence, the working group endorsed the following lifestyle recommendations.
1.




Persons who would benefit from LDL-C lowering should consume a dietary pattern that emphasizes intake of vegetables, fruits, and whole grains and includes low-fat dairy products, poultry, fish, legumes, non-tropical vegetable oils, and nuts; examples include the DASH diet, the USDA food pattern, or the AHA diet. Saturated fats (aim for <6 % of calories) and trans fats should be minimized.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


