Fig. 1.1
The apex of the heart when viewed from above in systole and diastole; note the position of the valves and their relationships
The heart is a muscular organ; its location is posterior to the sternal bone in the anterior mediastinum, a bit deviated to the left. Anatomically speaking, the heart is composed of three layers:
“Pericardium”: the outermost layer, covers the heart as a tissue sac, and has itself three layers:
1.
Fibrous pericardium (firm, outermost layer).
2.
Parietal pericardium (between fibrous pericardium and visceral pericardium).
3.
Visceral pericardium (innermost layer of pericardium) which is attached directly to the outer border of myocardial tissue; normally, a potential space exists between visceral and parietal pericardial layers which are filled with a few milliliters of serous tissue, functioning as a lubricant between the two layers while there is continuous heart rhythm and myocardial contractions.
“Myocardium”: the middle layer, has the main role of contraction, and is composed mainly of:
1.
Myocardial muscle tissue
2.
Coronary vascular system
“Endocardium”: the innermost layer, covers the inner space of the cardiac chambers
(Silver et al. 1971; Anwar et al. 2007; Tops et al. 2007; Haddad et al. 2009; Silbiger and Bazaz 2009; Ho and McCarthy 2010; Rogers and Bolling 2010; Atkinson et al. 2011; Dell’Italia 2012; Silbiger 2012)
Here we discuss more about the myocardial muscle tissue and its ingredients. The cardiac muscle (myocardium) is mainly composed of three cell types:
1.
Cardiac connective tissue cells
2.
Cardiomyocytes (which have contractile function)
3.
Cardiac electrical system cells (consisting of “impulse-generating cells” and “specialized conductive cells”)
1.1.1.1 Cardiac Connective Tissue Cells
The cardiomyocytes are arranged in a cellular bed of protective system and supporting structure known as the cardiac connective tissue cells; these cells have the following main functions:
1.
Supporting the cardiac muscle fibers as a physical protective structure
2.
Transmission of the cardiomyocyte-produced mechanical force to cardiac chambers
3.
Adding “tensile strength and stiffness” to the structure of the heart
4.
Preventing excessive dilation and overexpansion of the heart
5.
Keeping the heart within its original framework, returning the heart to its original shape after each contraction through the elastic fibers
The cardiac connective tissue would be modified according to the function of the related cardiac region; for example, “the amount of collagen in atria is different than in the ventricles” which shows the diversities and dissimilarities of anatomy that are the result of difference in function, both regarding “pressure and volume” work of different cardiac regions (Borg et al. 1982; Robinson et al. 1986, 1988; Rossi et al. 1998; Distefano and Sciacca 2012; Watson et al. 2012).
1.1.1.2 Cardiac Contractile Tissue Cells (i.e., Cardiac Muscle Cells or Cardiomyocytes)
The following hierarchy could lead us to overall order seen in the fine and specialized structure of the myocardial histology:
The myocardium is composed of myocardial cells called heart muscle cell, cardiac myocytes, or, briefly, cardiomyocytes.
These cells have contractile function similar to striated muscle cells, with the especial difference that their contraction is involuntary.
Also, instead of having many nuclei in each cell (like the cellular structure seen in skeletal muscle cells), each cardiomyocyte has only 1–2 nuclei and is 100 μ in length and 25 μ in width.
The internal structure of each cardiomyocytes is in turn composed of a wealth of cardiac myofibrils.
And finally, each cardiac myofibril is composed of a vast number of sarcomeres; each sarcomere is located anatomically between two Z lines; thin filaments are attached perpendicularly to Z lines on each side, while thick filaments are in between them in a parallel fashion (Fig. 1.2).
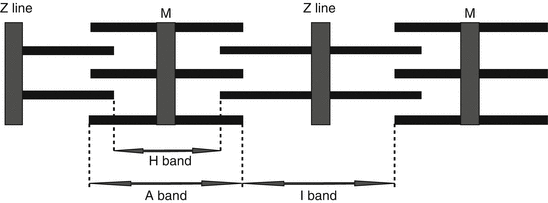
Fig. 1.2
Microscopic structure of a sarcomere; thin and thick filaments are presented as thin and thick interspersed horizontal rods; a sarcomere is defined as the part of sarcomere between two Z lines
Now let’s discuss the above lines in more detail.
The cardiomyocytes are specialized muscle cells, ranging from 25 μm length in atria up to about 140 μm in ventricular cardiomyocytes. About half of a cardiomyocyte is composed of contractile parts (called myofibrils) arranged as contractile units called sarcomere (each cardiomyocyte contains a number of sarcomeres); sarcomere is the basic unit of contraction or better to say contractile quantum of the heart.
The other half is composed of other cellular structures including nucleus, mitochondria, sarcoplasmic reticulum, and cytosol.
Sarcolemma, T tubules, and sarcoplasmic reticulum: each cardiomyocyte is enveloped by a especial membrane called sarcolemma, which not only covers the cardiomyocyte but also has a large network “invaginating” between the cells creating transverse tubules (T tubules) having a central role in Ca2+ transfer in sarcoplasmic reticulum of the cardiomyocytes. Ca2+ has a pivotal role in all the main three cardiac physiologic functions, known among them is excitation-contraction coupling which is discussed later; however, in summary, excitation-contraction coupling could be assumed as the “hinge” between the electrical and mechanical functions of the cardiomyocyte.
The sarcoplasmic reticulum (SR) has a dual function for Ca2+ homeostasis; first, SR releases Ca2+ after Ca2+ influx during depolarization, causing contractility through junctional SR (JSR), and after that, SR reuptakes Ca2+ causing cardiac muscle relaxation through longitudinal SR (LSR).
Intercalated discs: intercalated discs are among the basic cellular structures found in cardiomyocytes, which are “cardiac-specific structures”; these cardiomyocytes structures are the main communication port between adjacent cardiomyocytes.
The main functions of intercalated discs could be categorized as:
1.
Mechanical connection between adjacent cardiomyocytes
2.
Electrical transport between adjacent cardiomyocytes (i.e., rapid transduction and transmission of action potential)
3.
Synchronization of cell contraction
The above main functions of the intercalated discs have an integral role in creating a “physiologic” syncytium. Intercalated discs are special to cardiac muscle cells; adult skeletal muscle cells are devoid of these specialized cellular structures. Intercalated discs perform their roles through three types of intercellular junctions:
1.
Spot desmosomes
2.
Sheet desmosomes
3.
Gap junction
Spot desmosomes are intercellular connections which “anchor the intermediate-filament cytoskeleton” in the adjacent cells.
Sheet desmosomes are the place for contractile structures that connect two neighboring cells; it means that sheet desmosomes fasten and fix the contractile apparatus between the neighboring cells.
Gap junctions are primarily responsible for electrical transmission between adjacent cells causing rapid electrical wave progression in “cardiac syncytium” having two roles:
Anchorage which is an integral part of cardiac morphogenesis
Communication which is essential for cardiac conduction and cardiac action potential propagation
Gap junctions are composed of connexins (mainly connexin 43) as one of their main subunits, so the cellular pathologies in gap junctions of cardiomyocytes (especially those related to connexin 43) can have a major role in ischemia and some lethal arrhythmias. In human, connexin 43 is the most common and important type of cardiac connexins. Usually, the Purkinje cells have a high amount of gap junctions, while they do not have considerable amounts of contractile elements.
Each cardiomyocyte is composed of a number of contractile units: let’s say contractile quantum or as we are more familiar it is called cardiac sarcomere. So, sarcomere is the basic unit of contraction (i.e., the contractile quantum of the heart). The primary function of cardiomyocyte is produced in each sarcomere.
As mentioned above, the cardiomyocytes are ranging from 25 to 140 μm in diameter; meanwhile, cardiac sarcomeres are “contractile quantum” of the heart and are about 1.6–2.2 μm in length.
Nearly about half of each sarcomere is composed of contractile elements, arranged as contractile fibers, while the other half is composed of all other cellular structures like mitochondria, nucleus, cytosolic structures, and other intracellular organelles.
The contractile fibers are classically divided as thick filaments and thin filaments; however, if the microscopic anatomy of sarcomere is viewed, each sarcomere is defined as the contractile part of the sarcomere located between two Z lines and consists of the following parts:
Z lines: when seen with a microscope present as thick lines, the margins of each sarcomere is defined by Z line in each side; Z stands for “Zuckung,” a German name meaning “contraction” or “twitch”; so, each sarcomere is the region of myofilaments between two Z lines; the Z line is like an “anchor” to which the thin filaments are attached.
Thin filaments are attached perpendicularly to Z lines on each side; thin filaments are composed of actin, tropomyosin, and troponin.
Thick filaments are in between them in a parallel fashion; these filaments are composed of myosin and are located in the center of the sarcomere; the two ends of thick filaments are interspersed with thin filaments.
“I” band is the area of sarcomere adjacent to Z line; during myocardial contraction, “I” band shortens.
“A” band is the central part of each sarcomere; each “A” band, while located in center, takes two “I” bands (each I band in one side of the single A band) plus two Z lines (each Z line attached to the other side of “I” band); this complex composes a sarcomere (as presented in figure).
“H” band is the central part of “A” band, composed mainly of thick filaments.
A full description of contractile proteins, thick filament and thin filament, is described in this chapter in later sections and also in Figs. 1.2 and 1.6.
Histological differences between cardiac muscle and skeletal muscle: one could find the following differences between cardiac muscle cell and striated muscle cell.
Cardiac muscle tissue is a complex of united and combined contractile cells, totally named as a syncytium; this syncytium is:
Composed of branched cells with the myofibers usually being fused at their ends.
Connected together through a relatively unique cardiac cellular structure called intercalated discs.
Electrical current is transmitted by an especial electrical link “gap junctions.”
Cardiomyocytes usually have 1 or 2 (rarely 3–4) central nuclei.
Accompanied with many mitochondria having an essential role in energy production and metabolism regulation, the energy is delivered as ATP through oxidative phosphorylation for many processes including “excitation-contraction coupling” and the “sarcomere activity” and the relationship between contractile filaments in systole and diastole.
One of the most important functions of mitochondria is Ca2+ homeostasis (see below); this is why in cardiomyocytes, the mitochondria are located near the sarcoplasmic reticulum (SR).
Both mitochondria and Ca2+ have a central role in cardiomyocyte necrosis; the role of mitochondria changes from an “ATP-producing engine” to “producers of excessive reactive oxygen species” which would release “pro-death proteins.”
The high rate of metabolism in these cells necessitates high vasculature with all the cells having aerobic metabolism.
The special Ca2+ metabolism of these cells is the main result for having fewer T tubules, while these T tubules are wider (cardiac T tubules are about 5 times more than skeletal muscles in diameter).
Thin filaments in cardiac muscles do not have a constant length.
Skeletal muscle cells have the following features due to their pattern of contraction; which is a pattern of neuromuscular junction unit:
Longer, multinucleated, and cylindrical shape.
Usually not arranged as syncytium; instead, they are located side by side with no tight binding or gap junctions.
Lower metabolism needs necessitating medium vasculature, with lower amounts of mitochondria (about 2–3 % of the cell).
Both aerobic and anaerobic metabolism.
Thick and thin filaments in skeletal muscles have a constant length
(Severs 1985; Peters 1996; Gordon et al. 2000; Kirchhoff et al. 2000; Lo 2000; Alberts 2002, 4th edition, New York: Garland Science; Burgoyne et al. 2008; Kobayashi et al. 2008; Meyer et al. 2010; Shaw and Rudy 2010; Workman et al. 2011; Anderson et al. 2012; Balse et al. 2012; Bingen et al. 2013; Delmar and Makita 2012; Eisner et al. 2013; Khan et al. 2012; Kubli and Gustafsson 2012; Miragoli et al. 2013; Orellana et al. 2012; Scriven and Moore 2013; Wang et al. 2012; Zhou and O’Rourke 2012).
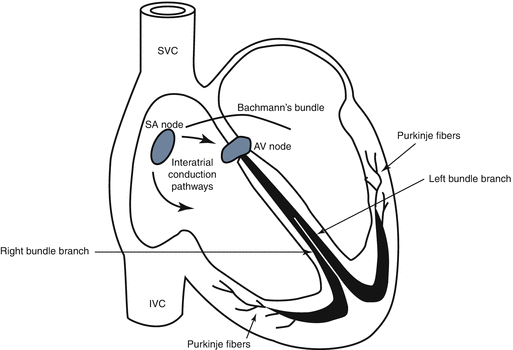
1.1.1.3 Cardiac Conductive Tissue Cells
The synchronized mechanical system needs a delicate electrical control known as cardiac electrical network or cardiac electrical system. Cardiac electrical system is composed of two main cells:
Excitatory cells known as “impulse-generating cells” consisting mainly of the sinoatrial (SA) node
Specialized conduction system known as “conductive cells” composed of the atrioventricular conduction pathways, AV node, the His bundle and its right and left branches, and finally, the Purkinje fiber cells or the Purkinje fiber network distributed all over ventricles to conduct the electrical impulse all over the ventricles effectively and rapidly
1.1.2 Anatomy of the Coronary Arteries
The coronary arterial system has four main elements (Fig. 1.4):
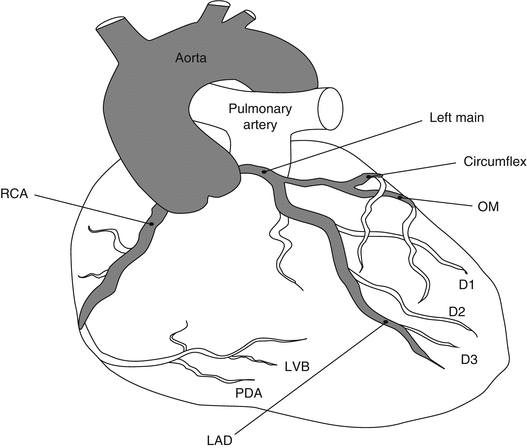

Fig. 1.4
Anatomy of normal epicardial coronary arteries
Left main coronary artery (LMCA)
Left anterior descending coronary artery (LAD)
Left circumflex coronary artery (LCX)
Right coronary artery (RCA)
1.1.2.1 Left Main Coronary Artery (LMCA)
LMCA starts from the left coronary ostium in left Valsalva sinus and after passing a length (between 0 and 40 mm) is divided to two branches: LAD and LCX. At times, an extra branch is divided from the LMCA and passes parallel to the diagonal arterial system; this arterial branch is called the “ramus” branch.
1.1.2.2 Left Anterior Descending (LAD) Artery
After LCX is separated from LMCA, the remainder of LMCA continues its path as left main coronary artery; LAD goes down the interventricular septum and reaches the apex:
The diagonal branches run as oblique derivations between LAD and LCX; the main role of diagonal branches is to perfuse the lateral wall of the left ventricle; these are demonstrated in Fig. 1.4 as D1 to D3.
Besides the diagonal branches, there are septal branches of LAD which perfuse the anterior two-thirds (2/3) of the interventricular septum.
1.1.2.3 Left Circumflex Coronary Artery (LCX)
LMCA is divided to LAD and LCX often at a 90° angle at the separation point; LCX has a number of ventricular branches which perfuse the lateral and posterior walls of the left ventricle (LV); these branches are called obtuse marginal or simply OM; in 40 % of the patients, LCX perfuses the SA node; the other 60 % are perfused by RCA.
1.1.2.4 Right Coronary Artery (RCA)
Right coronary artery (RCA) originates from the right coronary ostium of the Valsalva sinus; so, its origin is from a different coronary ostium compared with the abovementioned coronary arteries; RCA then goes through the right atrioventricular groove (i.e., the groove located between the atria and ventricles) towards right to reach the posterior part of interventricular septum where it gives a branch called acute marginal artery; as mentioned, 60 % are perfused by RCA. Finally, RCA is divided to two main branches:
Posterior descending artery (PDA): to perfuse the posterior 1/3 of the interventricular septum and the inferior wall of LV and also the posteromedial papillary muscle; in the majority of the people (85 %), PDA originates from RCA; these are called right dominant; however, in the other 15 %, called left dominant, PDA originates from LCX.
Posterolateral branch: to perfuse the posterior part of LV wall.
1.2 Cellular Physiology
Among the main characteristic features of cardiomyocytes are their very specialized functional and histological features; these subspecialized anatomical and physiological features have a key role in production, propagation, and transmission of “electrical and mechanical” functions of cardiomyocytes. Physiologically speaking, these electrical and mechanical functions are translated to three main domains:
1.
Action potential
2.
Excitation-contraction coupling (ECC)
3.
Contractile mechanisms and their related processes
As mentioned above, the heart muscle is composed of two main syncytia: the atrial syncytium and the ventricular syncytium. It means that in each syncytium, all the cells are interrelated with many widespread intercellular connections. The cardiomyocytes resemble the skeletal muscles, being composed of actin and myosin filaments, contracting and relaxing in a well-cooperated and organized manner in order to produce the cardiac contractile force. The intercellular connections between cardiac muscles are through the “intercalated discs” which are delicate pores located at the proximal and distal parts of each cardiomyocyte; these discs are able to transport great amounts of ions between the cardiomyocytes, transferring the ions from each cell to the next cell through the gap junctions. Hence, the term “syncytium” is not just an anatomical term but also a physiologic term. However, the two syncytia (atrial and ventricular) are separated physiologically by the AV node and AV bundle to act independently.
1.2.1 Action Potential
The normal cardiomyocytes have different electrical potentials known as action potentials. However, the resting potential and the action potential of all cells are not the same. Though, the production mechanism is similar and is the result of ion currents across the cellular membrane, the final result is consecutive depolarization and repolarization which produces the cardiac electrical impulse. The impulse is generated and conducted over the cardiac “electrical” and “conduction” system.
Action potential of cardiomyocytes is composed of five phases which are produced due to the influx and efflux of ions; especially Na+, Ca2+, and K+ ions, across the cell membrane.
This action potential is about 105 millivolts (mV) starting from about −80 to −90 mV reaching up to +15 to +20 mV, then experiencing a plateau for about 0.2 milliseconds, and finally turning down to the baseline which is −80 to −90 mV (Eisner et al. 2013).
The cardiomyocyte action potential is much similar to the action potential of skeletal muscle; however, it has two main features:
First of all, the fast Na+ channels are present, both in the skeletal muscles and the cardiomyocyte.
Second, the slow (L)-type Ca2+ channels are present in the cardiomyocytes, but not in the skeletal muscle cells; however, after the start of action potential mainly by the fast Na+ channels, L-type Ca2+ channels would open late and also would remain open for a few milliseconds to create the plateau of action potential. These channels have two main effects: first to decrease the heart rate in the physiologically defined range and second to augment cardiomyocyte contractions.
Besides Na+ and Ca2+, the third important ion in cardiomyocyte action potential is K+. Just after cardiomyocyte depolarization, due to Ca2+ entry to the cell, there is abrupt and considerable decreases in K+ outflux from the cell to the external milieu. This is also an important reason for delayed plateau of the action potential, mainly created by the slow (L)-type Ca2+ channels but also enforced by K+ outflux. The permeability of the cardiomyocyte cell membrane to K+ will return to normal after cessation of Ca2+ and Na+ channels to normal potential (about 0.2–0.3 milliseconds) which causes the return of K+ outside the cell and ending action potential.
The phases of action potential in ventricular and atrial cardiomyocytes and also His bundle and Purkinje cells are:
Phase 0: early rapid upstroke of action potential caused by huge Na+ influx.
Phase 1: short-term and incomplete repolarization due to K+ outflux.
Phase 2: slow (L)-type Ca2+ channels open and there is Ca2+ influx; initiation of the contractions starts immediately afterwards; this phase is also called plateau.
Phase 3: large amounts of K+ outflux which overcome the Ca2+ influx; again the action potential moves to negative levels to reach the resting potential; this phase, named resting potential phase, equals diastole.
Phase 4: influx of very negligible amounts of K+; however, the “Na+-Ca2+ exchanger” also known as “NCX” has a very important role in relaxation phase, since it sends Ca2+ against its gradient into the exterior of myocardial cell and sends K+ against its gradient to interior of myocardial cell; the failure of this pump to function properly has been implicated as one of the mechanisms involved in heart failure (Table 1.1, Fig. 1.5).
Table 1.1
A summary of action potential events in ventricular and atrial cardiomyocytes and also His bundle and Purkinje system
Phase
Term
Involved ion(s)
0
Rapid upstroke
Na+ influx
1
Short-term and incomplete repolarization
K+ outflux
2
Plateau
Ca2+ influx and K+ outflux
3
Repolarization (main part)
Large K+ outflux
4
Diastole (resting potential)
K+ influx (very negligible amounts)
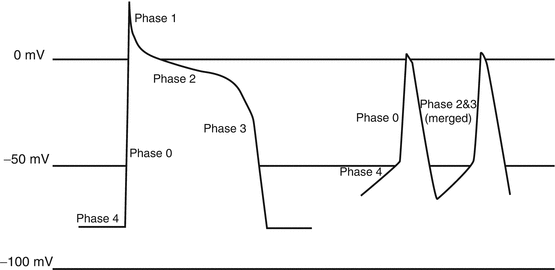
Fig. 1.5
Action potential in a normal cardiac cell (left) and a conducting cell (right)
There are a number of differences between “sinoatrial (SA) node and atrioventricular (AV) node cells” on one side with “ventricular and atrial cardiomyocytes and also His bundle and Purkinje cells” on the other side regarding the phases of action potential; these differences are mainly due to increased “Na+ influx” during phase 4 and increased “Ca2+ influx” and decreased “K+ influx” during phase 4 which causes:
Resting potential of pacemaker cells is less negative than the other cardiac cells; it means that if resting potential in majority of cardiac cells is −80 to −90 mV, it would be just −50 to −60 mV in pacemaker cells; the reason for the change is that the flux of Na+ from outside to inside of pacemaker cells (i.e., Na+ influx) continues during repolarization, changing the resting potential level from about −90 mV to upper levels in such a way that it reaches the needed voltage for threshold of repolarization (i.e., about −50 to −60 mV); this Na+ current is called pacemaker funny current of heart, i.e., I(f) of the heart; this funny current is responsible for rhythmic, spontaneous, pacemaker activity of the pacemaker cells, especially SA node.
Phase 4 (diastole) of action potential is more abrupt and head up, i.e., not as much flat of the cardiac muscles’ action potential, again due to the same Na+ current in repolarization period.
Phase 1 of action potential is nearly eliminated.
Phases 2 and 3 are nearly merged together.
Refractory period of cardiomyocytes: refractory period in cardiac action potential is the time interval after termination of each action potential in which no new impulse could be generated after any stimulus; the role of refractory period is to prevent premature contractions during a definite time interval and also could have a protective role for the heart against “reentrant arrhythmias.” However, the time interval for refractory period is not constant all over the cardiac cells, being shorter in the atrial cells (0.15 s) than the ventricular cells (0.3 s). Physiologically speaking, the phase 2 (plateau) of action potential is the main determinant factor for duration of refractory period.
(Boyden et al. 1988; Szigligeti et al. 1996; Reuter et al. 2005; DiFrancesco 2006, 2010; Bucchi et al. 2007; DiFrancesco and Borer 2007; Zhang and Hancox 2009; Chen et al. 2010; Neco et al. 2010; Pott et al. 2011; Coronel et al. 2012; DiFrancesco and Noble 2012; Ednie and Bennett 2012; Shy et al. 2013; Strege et al. 2012; Torres-Jacome et al. 2013; Brunello et al. 2013; Goldhaber and Philipson 2013; Kim et al. 2013; Ottolia et al. 2013; Papaioannou et al. 2013; Sipido et al. 2013; Weisbrod et al. 2013)
1.2.2 Excitation-Contraction Coupling (ECC)
Excitation-contraction coupling (ECC): this term, used in 1952 for the first time, depicts a physiologic process which transforms an electrical impulse to a mechanical contraction which is seen in both skeletal and cardiac muscles. In cardiac muscles, ECC acts as a “joint” in cardiomyocytes between the electrical function and mechanical function of the heart. ECC is one of the most important mechanisms in cardiac physiology. This very important mechanism is composed of three main delicate cellular and subcellular mechanisms, each having their individual elements; when these structures interact together in a regulated manner, the electrical action potential is changed to the mechanical force of the cardiomyocyte. These composing aspects are:
1.
Functioning organelles of ECC
2.
Calcium ion (Ca2+)
3.
Controllers of ECC
A summary of these composing aspects and their related items are presented in the Table 1.2.
Table 1.2
A summary of the composing aspects of ECC and their related items
1 | Functioning organelles of ECC | Cell membrane |
Thick and thin filaments | ||
T tubules | ||
Sarcoplasmic reticulum | ||
2 | Calcium ion (Ca2+) | Ca2+ influx to the cardiomyocytes (by L-type Ca2+ channels in systole) |
Ca2+ release inside the cell (by RyR in systole) | ||
Ca2+ efflux from the cardiomyocytes (by NCX in diastole) | ||
Ca2+ reuptake from the cell (by SERCA in diastole) | ||
3 | Controllers of ECC | Ryanodine receptor (RyR) family |
Dihydropyridine receptor (DHPR) | ||
Calmodulin |
1.2.2.1 Which Parts of Cardiomyocyte Are the Functioning Organelles of ECC?
Which parts of cardiomyocyte are the main components of ECC? The following parts of cardiomyocyte involved in the ECC process are:
1.
Cell membrane (which is responsible for electrical function, i.e., action potential; discussed before)
2.
Thick and thin filaments (which are responsible for mechanical function, i.e., contractile function; discussed later in this chapter)
3.
Mitochondria (ECC needs a great amount of energy; mitochondria are responsible for supporting ECC regarding its energy needs in the form of ATP through oxidative phosphorylation; discussed before)
4.
Sarcoplasmic reticulum (known as SR; discussed here)
5.
Transverse tubules of cardiomyocytes (known as T tubules; discussed here)
Sarcoplasmic reticulum: SR is divided into longitudinal SR (LSR) and junctional SR (JSR). LSR releases Ca2+ reserves into the cell as fast as possible in just a few milliseconds, which would activate cardiomyocyte contractile structures. Junctional SR contains huge “Ca2+-releasing channels” called “ryanodine receptors.” These receptors form a protein network which would enhance the release of Ca2+ in response to the Ca2+ influx. The role of “ryanodine receptors” is more recognized when considering this fact:
T tubules: as mentioned before, T tubules are invaginations of the cardiomyocyte cell membrane into the interior space of the cardiac muscle cells and transmit the action potential of the cell membrane to the interior parts of the cardiomyocyte. The role of T tubules is conducting the depolarization phase of action potential, as rapidly as possible, from the cell membrane to the interior of the cell.
For cardiac cell contraction, nearly 75 % of Ca2+ in cardiac cell cytoplasm is released from SR.
Then, the electrical current produced by action potential is transmitted through the T tubules to the interior of the cell, to the “longitudinal sarcoplasmic reticulum.” During some cardiac diseases like heart failure or ventricular hypertrophy, the “loss of integrity in transverse tubules” is one of the main etiologies for impaired availability of Ca2+ for sarcomere contractile mechanisms, which would impair Ca2+ movements and its availability for contraction of the sarcomere myofilaments.
T tubules of cardiomyocytes have some unique features:
1.
Ca2+ is the main mediator playing the most important role in cardiomyocyte action potential, ECC, and finally, muscle contraction. Although the start of action potential in cardiac muscles is similar to skeletal muscle, its continuation is dependent on the role of Ca2+, as mentioned above (see subtitle of action potential). As mentioned above, the role of Ca2+ is also important in the release of intracellular Ca2+ reservoirs: “the CICR phenomenon”; CICR is one of the mechanisms demonstrating why structural disintegration and disturbance of T tubules is an early happening in heart failure.
2.
Although cardiac cell action potential is the main trigger for Ca2+ release, the first Ca2+ release is from the large Ca2+ reservoirs of T tubules, and T tubules would trigger the release of more Ca2+ from SR. As mentioned before, the influx of Ca2+ from ECF to interior of cardiac cells through slow (L)-type calcium channels located on the T tubule strengthens the depolarization of cardiac muscle cells and causes the plateau phase of depolarization; this feature is special to depolarization of cardiac cells, while in skeletal muscle depolarization, the influx of Ca2+ to skeletal cells, through T tubule’s slow (L)-type calcium channels, does not have any significant role.
3.
T tubules are invagination of the cell membrane to the cells; it means that T tubules are in fact part of the extracellular fluid (ECF); so, they have continuous exchange of Ca2+ with ECF. Any decrease in Ca2+ concentration of blood would be associated with a decrease in Ca2+ concentration of ECF, which in turn would reduce Ca2+ concentration in the intracellular milieu; this is why any decrease in plasma levels of Ca2+ is associated with decreased cardiac contractility.
1.2.2.2 Ca2+ Homeostasis
Ca2+ homeostasis in cardiomyocytes is such an important issue that any perturbation in its equilibrium state would result in cardiac disturbances. Intracellular Ca2+ is considered as a second messenger in cardiac sarcomeres, while its concentration and trends of change exert important effects on “mitochondrial energy,” “cell death or apoptosis,” and “the intracellular buffering capacity for controlling stress.”
To have this equilibrium in a continuous manner for a lifelong time, a delicate balance between Ca2+ influx and Ca2+ efflux in cardiomyocytes is an obligation: the Ca2+ balance has a central role in each cardiac cycle, composed of a systole (contraction) and a diastole (relaxation); although there are a number of states in which influx would exceed efflux or vice versa, a number of subcellular mechanisms work together to modify these fluxes and reach the final equilibrium in such a way to increase the efficacy of myocardial contractions as a result of control in Ca2+homeostasis.
Ca2+ surge and Ca2+ reuptake are both located inside the cardiomyocytes and also, both are among the main features of systole and diastole, respectively. This dual phase is seen in all aspects of Ca2+ homeostasis, including cardiac contraction, Ca2+ flow direction, Ca2+ concentration inside each cell, and Ca2+ release and reuptake in all potential intracellular elements like mitochondria.
The dual phase pattern of Ca 2+ with its widespread pattern of distribution and its extensive effects is controlled mainly by four mechanisms:
1.
Ca2+ influx to the cardiomyocytes (mainly by L-type channels in systole: contraction phase)
2.
Ca2+ release inside the cell (by ryanodine receptor or “RyR” in systole: contraction phase)
3.
Ca2+ efflux from the cardiomyocytes (mainly by Na+-Ca2+ exchanger “NCX” in diastole: relaxation phase)
4.
Ca2+ reuptake from the cell (by sarcoendoplasmic reticulum Ca2+ transport ATPase “SERCA” in diastole: relaxation phase)
One of the main etiologic mechanisms for heart failure is “reduced and sluggish Ca2+ release and slow removal of Ca2+.” In these patients, reduced and delayed function of L-type Ca2+ channel, slowed release of Ca2+ from SR, and “delayed activation” of Na+-Ca2+ exchanger “NCX” are among the most important mechanisms involved in the pathogenesis of the disease state.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree