Long-term cardiac pacing
An artificial cardiac pacemaker generates electrical stimuli that can initiate myocardial contraction. The stimuli are delivered to the heart by transvenous leads or much less commonly via epicardial electrodes. (Currently, a leadless miniature pacemaker inserted percutaneously into the cavity of the right ventricle is being developed!)
The first pacemaker was implanted in 1958. Rapid progress in technology and an increasing awareness of the benefits of pacing have led to pacemakers being widely used. People of all ages, from the newborn to patients over 100 years old, have been paced.
Common indications for long-term pacing
There are international guidelines for the indications for pacemaker implantation, some of which the author has found somewhat difficult to interpret! The indications below are largely in accordance with those guidelines.
Complete atrioventricular block
Syncope
The most common reason for pacemaker implantation is to prevent recurrence of syncope or near-syncope due to complete atrioventricular (AV) block. A single episode is a sufficient indication, and since the next blackout may cause injury or be fatal, delay should be minimal. Even in patients with a short life expectancy, pacing should be considered if by preventing syncope independence may be preserved and injury avoided.
Dyspnoea and heart failure
Complete heart block can reduce cardiac output and thereby cause exertional dyspnoea and sometimes cardiac failure.
Prognosis
Without pacing, the prognosis in patients with complete heart block is poor. With an artificial pacemaker, life expectancy closely approaches that of the general population, though those with overt coronary heart disease or with heart failure have a less good outlook.
Pacemaker implantation should be considered in asymptomatic patients with complete AV block, particularly when the ventricular rate is 40 beats/min or less, on purely prognostic grounds. Furthermore, by preventing a first syncopal episode, pacing may well prevent major injury to the patient.
QRS breadth
Narrow ventricular complexes during complete AV block suggest that interruption in conduction is at the AV nodal level and that, in contrast to infranodal block, a subsidiary pacemaker within the bundle of His will discharge reliably at a relatively rapid ventricular rate. However, in practice, patients with narrow ventricular complexes during complete heart block often experience syncope and impaired exercise tolerance and do require pacing.
Congenital heart block
Congenital heart block, i.e. complete AV block that is discovered when the patient is a neonate or child and is not caused by acquired disease, is widely regarded as benign. This is incorrect. Patients do develop symptoms and can die suddenly. If heart block has caused symptoms then pacing is indicated.
In young asymptomatic patients the risks of not implanting a pacemaker have to be weighed against the possibility of complications associated with several decades of pacing. There are a number of documented risk factors: day-time ventricular rate less than 50 beats/min, broad QRS complexes, pauses more than 3.0 s, frequent ventricular ectopic beats and poor chronotropic response to exercise. Unpaced patients should undergo ambulatory and exercise electrocardiography at regular intervals. In older patients who present with congenital heart block, the threshold for implanting a pacemaker should be low.
Neuromuscular diseases
Pacing is indicated in neuromuscular diseases such as myotonic muscular dystrophy, limb-girdle muscular dystrophy and peroneal muscular atrophy, if there is high-degree AV block, because of the risk of sudden death. Indeed, pacing should be considered with any form of AV block with or without symptoms, because of the high risk of rapid progression to complete AV block.
Second-degree atrioventricular block
Mobitz II AV block often progresses to complete AV block. The management of Mobitz II is the same as that for complete AV block.
A study has refuted the previously held view that Mobitz I (Wenckebach) AV block is benign, in that the incidence of symptoms, prognosis and benefits of pacing were the same as for patients with Mobitz II block. However, Mobitz I block in young people with transient and often nocturnal Wenckebach block is due to high vagal tone. It is benign and pacing is not indicated. Adult patients who are found to have sustained periods of AV Wenckebach block during the daytime should be considered for pacing unless they undertake a lot of physical training, in which case their AV block may be attributable to high vagal tone.
First-degree atrioventricular block
First-degree AV block is not usually an indication for cardiac pacing. If a patient presents with first-degree block and syncope it is quite possible that the symptoms are due to transient second- or third-degree AV block, but a pacemaker should not be implanted without proof of this, e.g. by ambulatory electrocardiography.
Exceptionally, the PR interval is so long that the P wave immediately follows the preceding QRS complex. This may lead to the equivalent of the ‘pacemaker syndrome’ (see below), in which case dual-chamber pacing is indicated.
Bundle branch and fascicular blocks
Bundle branch block
The risk of high-degree AV block developing in an asymptomatic patient with either left or right bundle branch block is relatively small (Chapter 4), and pacing is not indicated. In patients who present with syncope or near-syncope, confirmation of intermittent high-degree AV block should be sought.
Bifascicular block
In bifascicular block the remaining functioning fascicle may fail to conduct, intermittently or persistently, and cause high-degree AV block. In patients with a typical history of Stokes–Adams attacks, pacemaker implantation is indicated to prevent syncope without further investigation. With atypical symptoms, high-degree AV block must be documented first. It should be borne in mind that some patients with bifascicular block have been shown to be prone to ventricular tachycardia.
In asymptomatic bifascicular block, the chances of progression to complete AV block is in the order of 2% per year, and the major determinants of prognosis are the presence of coronary artery or myocardial disease; prophylactic pacing is generally not indicated. Additional first-degree AV block or bundle of His electrographic evidence of prolonged infranodal (HV) conduction suggests that conduction in the functioning fascicle is also impaired. Guidelines do recommend that patients with bifascicular block who are found to have a markedly prolonged HV interval at His bundle electrography (Chapter 25) are considered for pacing.
Alternating bundle branch block
‘Alternating bundle branch block’, i.e. alternating patterns of left and right bundle branch block or, in patients with right bundle branch block, alternating left anterior and posterior fascicular block, is an indication for pacing.
Atrioventricular and bundle branch block after myocardial infarction
AV block due to inferior myocardial infarction usually resolves within a few days and almost always by three weeks. When anterior infarction is complicated by high-degree AV block, there is usually extensive myocardial damage and hence the prognosis is poor. Though block may persist, it is prudent to ensure that the patient is going to survive before implanting a pacemaker. Thus, pacemaker implantation should not be considered unless second- or third-degree AV block is present three weeks after either inferior or anterior myocardial infarction.
Bifascicular block persisting after acute anterior infarction complicated by a period of AV block raises the possibility that complete AV block might recur. However, there is little evidence that prophylactic pacing reduces mortality.
When a patient is admitted to hospital with heart block, there is often an unnecessary delay before referral for long-term pacing while myocardial infarction is excluded. Unless the patient has experienced typical cardiac pain or there are typical ECG changes of recent infarction, it is very unlikely that AV block has been caused by acute infarction, and delay should be avoided.
Sick sinus syndrome
Syncope
Sick sinus syndrome accounts for more than one-third of pacemaker implantations. Pacing is indicated when syncope or near-syncope are caused.
It should be remembered that sinus bradycardia and pauses in sinus node activity for up to 2.0 s, particularly if nocturnal, can be physiological.
Bradycardia–tachycardia syndrome
In patients with the bradycardia–tachycardia syndrome, pacing may be required to avoid severe bradycardia caused by antiarrhythmic drugs. Atrial tachyarrhythmias which start during bradycardia may be prevented by atrial pacing.
Prognosis
Pacing for sick sinus syndrome is not usually indicated in the absence of symptoms. However, daytime pauses in cardiac activity for several seconds might be considered an indication for pacing in those who operate machinery, including a motor car, to avoid an accident should syncope occur.
Hypersensitive carotid sinus and malignant vasovagal syndromes
Pacing will improve symptoms in these syndromes provided there is a significant cardioinhibitory component (Chapter 17).
Hypertrophic cardiomyopathy
Dual-chamber pacing with a short AV delay has been shown to reduce symptoms and left ventricular outflow tract gradient in some patients with hypertrophic cardiomyopathy who have a significant pressure gradient across the left ventricular outflow tract.
Resynchronisation therapy
Biventricular pacing can improve symptoms and also prognosis in patients with poor left ventricular function who have marked prolongation of QRS duration and impaired left ventricular function. Ventricular ‘resynchronisation’ is achieved by near-simultaneous delivery of stimuli conducted via right and left ventricular leads, usually resulting in significant shortening of QRS duration.
Left ventricular pacing is achieved by passing a lead into a lateral branch of the coronary sinus (Figure 23.24b). It can be technically challenging to introduce the lead into a suitable branch of the coronary sinus, achieve a satisfactory stimulation threshold and avoid diaphragmatic stimulation. Biventricular pacing is primarily to deal with cardiac failure rather than arrhythmia and therefore is not discussed in detail in this book.
Pacing modes
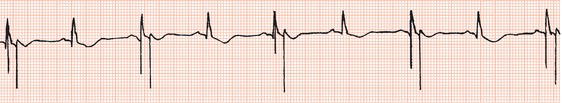
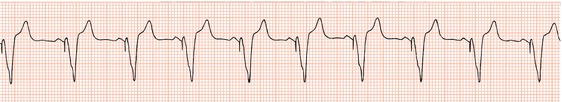
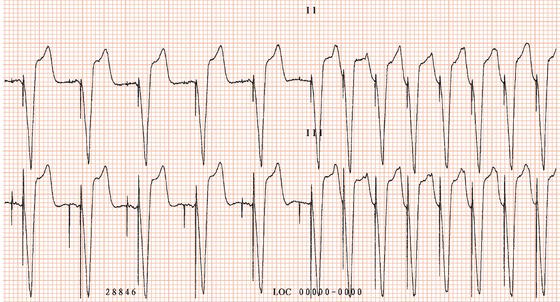
The first generation of pacemakers functioned in a fixed-rate mode. The pacemaker stimulated the ventricles repeatedly, usually at 70 beats/min, irrespective of any spontaneous cardiac activity (Figure 23.1). Competition with a spontaneous rhythm could cause irregular palpitation (Figure 23.2), and stimulation during ventricular repolarisation could possibly initiate ventricular fibrillation (Figure 23.3).
Figure 23.1 Fixed-rate ventricular pacing (leads I, II, III). A large pacing stimulus precedes each ventricular complex. P waves dissociated from ventricular complexes can be seen.
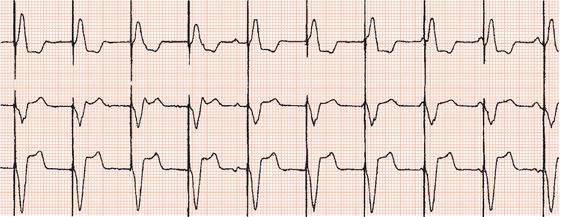
Figure 23.2 Fixed-rate ventricular pacing in a patient with first-degree AV block. The first three stimuli fall during the refractory period and are ineffective. The fourth causes a premature contraction.
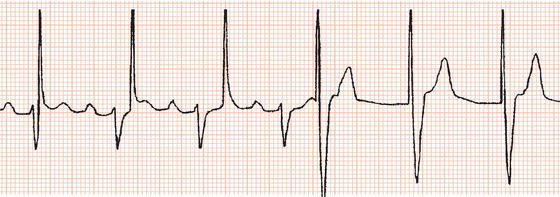
Figure 23.3 Examples of failure to sense. (a) Failure to sense in a demand ventricular pacemaker. The first, third, fifth and seventh pacing stimuli capture the ventricles. The second, fourth, sixth and eighth stimuli fall on the T waves of spontaneous ventricular beats. (b) Failure to sense the sixth ventricular complex, resulting in a pacemaker stimulus coinciding with the T wave and initiating ventricular fibrillation.
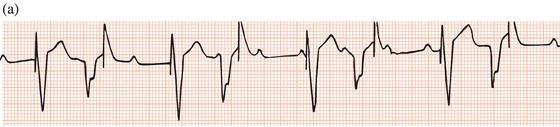
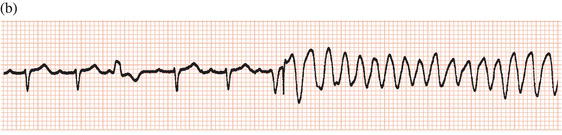
Subsequent developments enabled sensing of spontaneous activity via the stimulating lead to facilitate demand pacing. A sensed event resets the timing of delivery of the next pacemaker stimulus to avoid competition with spontaneous activity (Figure 23.4).
Figure 23.4 Demand ventricular pacemaker. The pacemaker is inhibited by the sinus beats (second and fourth complexes). The sixth complex is a fusion beat. A P wave can be seen to precede the pacing stimulus: by chance, a sinus impulse has arisen at the instant when the pacemaker was set to discharge and the ventricles have been activated by both the sinus node impulse and the pacemaker. Fusion beats should not be confused with failure to pace.
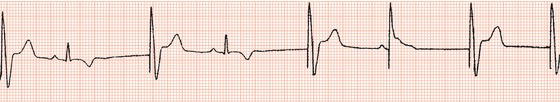
With the advent of reliable atrial transvenous pacing leads it became straightforward to pace and sense in the atrium as well as the ventricle, thus allowing atrial ‘single-chamber’ pacing and also ‘dual-chamber’ pacing, whereby stimulation and/or sensing can take place at both atrial and ventricular levels. These developments have facilitated a physiological approach to cardiac stimulation.
Pacing system code
A five-letter code is widely used to describe the various pacing modes, as shown in the table.
Single-chamber pacing
Ventricular demand pacing (VVI)
In the absence of spontaneous ventricular activity, a ventricular demand pacemaker, like a fixed-rate unit, delivers stimuli to the ventricles at a regular rate. However, if spontaneous activity is sensed via the ventricular lead, the timing of delivery of the next pacemaker output is reset to avoid competition.
In ventricular inhibited pacemakers (VVI) a sensed event terminates the current stimulation cycle, thus inhibiting pacemaker output, and starts a new cycle (Figure 23.4). The pacemaker is rendered insensitive immediately after a paced or sensed event for an interval which approximates the duration of myocardial activation plus recovery, to prevent sensing the ventricular electrogram which is produced by the event. This interval (250–300 ms) is referred to as the refractory period.
Ventricular demand pacing is a commonly employed mode, but its use has diminished now its disadvantages – the inability to facilitate the normal sequence of cardiac chamber activation and to provide a chronotropic response to exercise – are widely appreciated (see below).
Indications for ventricular demand pacing include bradycardia associated with persistent atrial fibrillation, second- and third-degree AV block in patients who are limited by impaired cerebral or locomotor function, and infrequent bradycardia when the pacemaker will be mainly on ‘standby’.
Figure 23.5 Atrial pacing. A pacing stimulus precedes each P wave. AV conduction is normal and hence each paced P wave is followed by a normal QRS complex after a normal PR interval.
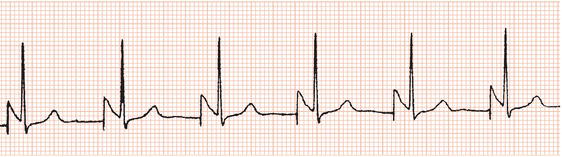
Figure 23.6 AV sequential (DVI) pacing. Pacing stimuli precede both atrial and ventricular complexes.
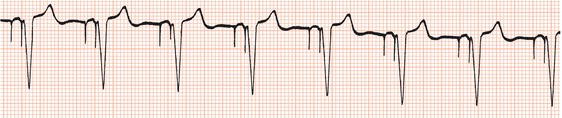
Atrial demand pacing (AAI)
The timing cycles of atrial inhibited (AAI) are the same as for ventricular demand pacing, as described above (Figure 23.5). With atrial pacing, the refractory period is usually longer, to avoid inappropriate inhibition of the pacemaker by sensing the ‘far field’ ventricular electrogram via the atrial lead.
Atrial pacing is indicated for treatment of the sick sinus syndrome unless AV conduction is impaired. By stimulating the atria rather than the ventricles, the normal sequence of cardiac chamber activation is maintained, loss of which can reduce cardiac output by up to one-third.
In patients with sick sinus syndrome, atrial pacing (including dual-chamber pacing, see below) has been shown to reduce the incidence of atrial fibrillation, heart failure and the pacemaker syndrome (see below) as compared with those patients in whom only the ventricles are paced.
Sick sinus syndrome can sometimes be associated with impaired AV conduction. However, if there is no evidence of it at the time of pacemaker implantation the subsequent development of impaired AV conduction is uncommon. Nevertheless, it is common practice to implant a dual-chamber pacemaker even though the risk of subsequent AV block is small. A dual-chamber pacemaker should be implanted if there is also bifascicular or bundle branch block, or if during pacemaker implantation, atrial pacing at a rate of 120 beats/min causes second-degree AV block.
Dual-chamber pacing
AV sequential pacing (DVI and DDI)
In AV sequential (DVI) pacing, the atria are stimulated first and then, after a delay that approximates the normal PR interval, the ventricles are stimulated (Figure 23.6). The pacemaker is inhibited by spontaneous ventricular activity but no sensing occurs in the atrium. As with other dual-chamber modes, both atrial and ventricular electrodes are required.
Fusion beats (Figure 23.7) are commonly seen during DVI pacing and are sometimes misinterpreted as pacemaker malfunction. Whereas the pacemaker is inhibited by an event sensed in the ventricles, the first chamber to be stimulated is the atrium. Pacemaker output may therefore occur at the same time as spontaneous atrial activation because its resultant ventricular depolarisation has not yet occurred.
Subsequently, DDI pacing was introduced, superseding DVI pacing. Sensing occurs at atrial as well as ventricular levels, thus avoiding competitive atrial pacing.
Unlike DDD pacing, sensed atrial events do not trigger ventricular stimulation, and thus DVI and DDI pacing will not facilitate endless loop tachycardia (see below).
The main indications for DVI and DDI pacing are sick sinus syndrome associated with impaired AV conduction, and carotid sinus and malignant vasovagal syndromes.
Atrial synchronised ventricular pacing (VDD)
In this mode, ventricular stimulation is triggered by a sensed atrial event after an interval similar to the normal PR interval (Figure 23.8). Thus the normal sequence of cardiac chamber activation is maintained and, provided sinus node function is normal, an increase in sinus node rate during exercise will lead to an increase in ventricular stimulation rate, i.e. a chronotropic response to exercise is facilitated.
If an atrial event is not sensed, ventricular stimulation continues at a fixed cycle length – otherwise atrial standstill might lead to ventricular asystole. To avoid atrial tachycardia or fibrillation triggering inappropriately fast ventricular pacing rates, there is an atrial refractory interval: the atrial channel is rendered insensitive during the AV delay and for a period after ventricular stimulation. Sensed atrial activity at a cycle length shorter than this period will not trigger ventricular stimulation.
The upper atrial rate that can trigger ventricular output is determined by the ‘total atrial refractory period’, which consists of the AV delay plus the post-ventricular stimulus refractory period. For example, if the AV delay is 125 ms and the atrial refractory period is 250 ms, the upper rate limit will be 60 000/375 = 160 beats/min.
In earlier years, sensing only took place in the atrium and pacing only in the ventricle (VAT). Thus, atrial activation would trigger ventricular activation irrespective of ventricular ectopic beats or ventricular rhythms faster than the sinus node rate. Subsequently, VDD pacing was introduced whereby sensing takes place in the ventricles as well so that spontaneous ventricular activity will inhibit the pacemaker (Figure 23.9).
VDD ventricular pacing is indicated in second- and third-degree AV block when sinus node function is normal. It is contraindicated in the sick sinus syndrome or when there are atrial tachyarrhythmias.
Figure 23.9 VDD pacing, showing chronotropic response to exercise (pacing rate = 98 beats/min) and inhibition of ventricular pacing by ventricular ectopic beats.
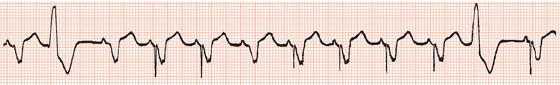
Endless loop tachycardia
If a ventricular stimulus is conducted retrogradely to the atria via either the AV junction or, if present, an accessory AV pathway, and the timing of the resultant atrial activation is outside the pacemaker’s atrial refractory period, it will trigger ventricular stimulation and hence initiate an ‘endless loop tachycardia’ (Figure 23.10), also referred to as ‘pacemaker-mediated tachycardia’.
Ventriculoatrial conduction is present in approximately two-thirds of patients with the sick sinus syndrome and one-fifth of those with complete AV block. Endless loop tachycardia can usually be prevented by prolongation of the atrial refractory period, but at the expense of reduction of the upper rate limit for ventricular stimulation. Endless loop tachycardia can be avoided in 90% of patients by setting the AV delay to 125 ms and the post-ventricular atrial refractory period to 300 ms.
Most pacemakers can automatically detect endless loop tachycardia and terminate it, for example by prolonging the atrial refractory period for one cycle.
Atrioventricular universal pacing (DDD)
In this mode (DDD), which all modern dual-chamber pacemakers can facilitate, both sensing and pacing can take place at atrial and ventricular levels, allowing the pacemaker to function in atrial demand (AAI), AV sequential (DDI) or atrial synchronised (VDD) modes, depending on the spontaneous heart rhythm (Figure 23.11).
If there is sinus bradycardia it functions as an atrial demand pacemaker. If there is impaired AV conduction, ventricular pacing is triggered either by spontaneous atrial activity or by delivery of an atrial stimulus. When sinus node function is normal, it functions in the atrial synchronised mode, thus providing a chronotropic response to exercise. The pacemaker is inhibited by both atrial and ventricular ectopic beats. Endless loop tachycardia may occur if there is retrograde AV conduction.
DDD pacing is indicated in second- and third-degree AV block.
Figure 23.11 Universal (DDD) pacing. In the first four beats, spontaneous P waves arising from the sinus node trigger ventricular stimulation. There is then sinus node slowing to which the pacemaker responds by pacing the atria as well as the ventricles (pacing stimuli can be seen preceding both the P waves and the QRS complexes).
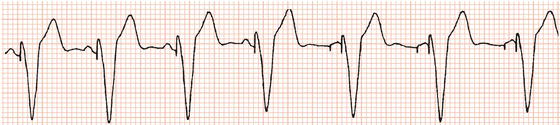
‘Physiological pacing’
Physiological pacing systems facilitate a chronotropic response to exercise by maintaining AV synchronisation as the sinus node rate varies, and/or by a rate-adaptive mechanism.
Atrial synchronised ventricular pacing (VDD, DDD)
VDD and DDD modes both maintain AV synchronisation and facilitate a chronotropic response to exercise.
Exercise capacity has been measured on a double-blind basis in patients with complete AV block during ventricular pacing (i.e. VVI) at 70 beats/min and during atrial synchronised ventricular pacing. The latter mode was shown to increase exercise capacity by approximately 30%.
Atrial synchronised pacing improves parameters in addition to exercise tolerance. Shortness of breath, dizziness and palpitation are less frequent, whereas fixed-rate pacing tends to impair the normal blood pressure response to exercise and leads to a higher respiratory rate and perceived exertion during submaximal exercise. The advantages of atrial synchronised pacing have been shown to be maintained long-term.
There are limitations to atrial synchronised ventricular pacing. First, normal or at least near-normal sinus node activity is required. Second, the ventricular stimulation rate may increase in response to an atrial tachyarrhythmia.
Rate-responsive systems
Several pacing systems are available that can facilitate a chronotropic response independent of atrial activity: a change in stimulation rate is achieved in response to a parameter that alters with exercise (see below). In contrast to atrial synchronised pacing, normal sinus node activity is not required.
In terms of exercise capacity, the ability to increase heart rate is far more important than maintaining AV synchronisation. This has been demonstrated by measuring exercise tolerance in patients with complete AV block during three pacing modes: fixed rate, atrial synchronised and ventricular pacing at a rate equal to but not synchronised with atrial activity. Both the latter forms of chronotropic pacing increased exercise performance to a similar degree as compared with fixed-rate pacing. Thus rate-responsive ventricular pacemakers can enable an enhanced exercise tolerance without AV synchronisation and in patients with atrial fibrillation.
Some patients with the sick sinus syndrome have chronotropic incompetence: there is little increase in sinus node rate in response to exercise. A rate-responsive system will facilitate an appropriate rate response.
According to the pacemaker code, atrial demand, ventricular demand and dual-chamber pacemakers with rate-response facilities are termed AAIR, VVIR and DDDR, respectively. All modern dual-chamber pacemakers can facilitate DDDR pacing.
Activity sensor
Vibration resulting from physical activity is sensed by a piezoelectric crystal attached to the inside of the pacemaker can or an accelerometer bonded to the circuitry within the pacemaker. The stimulation rate increases in parallel with the level of sensed activity. An accelerometer is regarded as more physiological, since it will respond to motion primarily in the anteroposterior direction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree