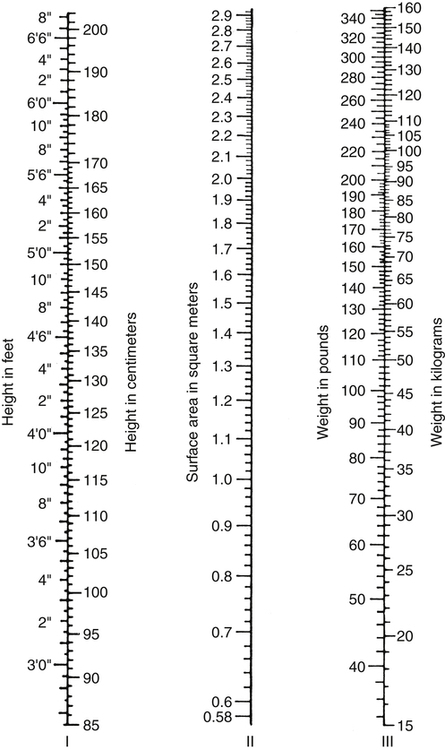
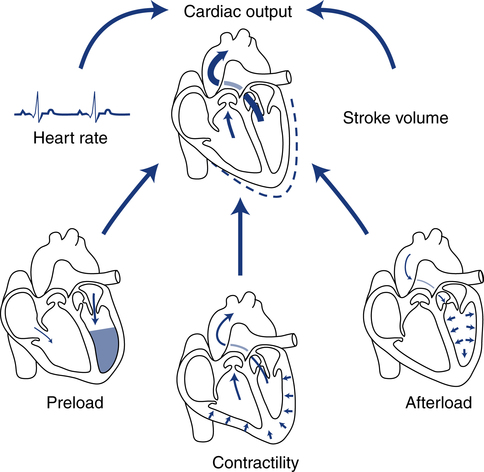
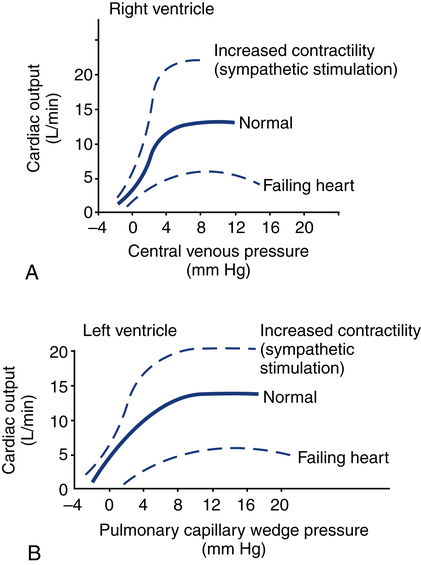
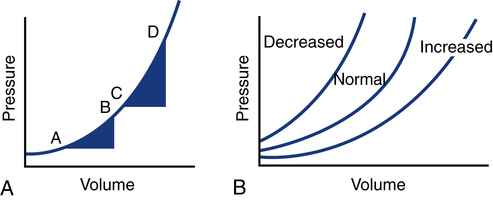
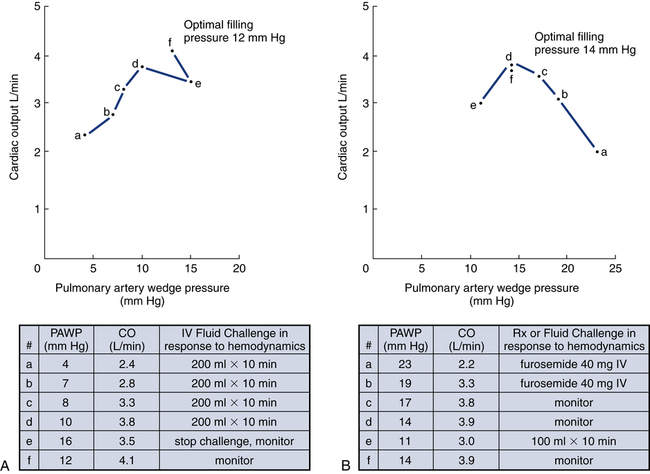
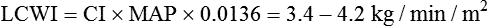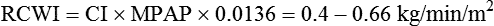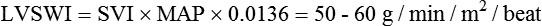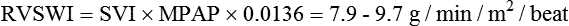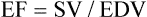After reading this chapter, you will be able to: 1. Define cardiac output, cardiac index, stroke volume, and venous return. 2. Describe the method of calculation, common reference range, and effect of sympathetic nervous stimulation on cardiac output. 3. Describe the effects of metabolism and reduced oxygen availability on the regulation of blood flow through organs. 4. Explain the effect of blood loss (hypovolemia) on circulatory function. 5. Describe the effect of mechanical ventilation on cardiac output and blood flow. 6. Explain the significance of the following indicators of cardiac output: 7. List the most important factors that regulate cardiac preload, afterload, and cardiac contractility. 8. Calculate systemic and pulmonary vascular resistance 9. Describe the technique for obtaining cardiac output through the following invasive methods: b. Thermodilution (pulmonary artery and transpulmonary) 10. Describe the noninvasive methods for evaluating cardiac performance: a. Transthoracic electrical bioimpedance c. Radionuclide cardiac imaging The aim of this chapter is to describe the physiologic principles used to measure cardiac output and the most common techniques used to assess it. Chapter 15 describes the specific vascular pressure parameters used in the intensive care unit (ICU) to monitor patients with circulatory issues. The amount of blood pumped out of the left ventricle in a minute is known as cardiac output (CO). It is the product of heart rate (HR) and stroke volume (SV), which is the volume of blood ejected by the ventricle by a single heartbeat (Box 16-1). Normal SV for adults is 60 to 130 mL/beat and is roughly equal for both the left and right ventricles. The average CO for men and women of all ages is approximately 5 L/minute at rest (reference range is 4 to 8 L/minute); however, the normal CO for an individual varies with age, sex (10% higher in men), body size, blood viscosity (hematocrit), and the tissue demand for oxygen.1 The normal heart is capable of pumping approximately 10 to 13 L/minute and twice that amount when stimulated by the sympathetic nervous system. A well-trained athlete’s heart enlarges sometimes as much as 50% and is capable of pumping up to 35 L/minute.1 Under normal conditions, the heart plays a passive role in CO and pumps whatever amount of blood is returned to it. When the diseased or damaged heart can no longer pump the amount of blood returned to it, it is said to be failing. In addition to providing tissue regulatory control of CO, the venous system acts as a reservoir of blood. Normally about two thirds of the total blood volume resides in the venous system. When blood volume to vital organs decreases, the veins and the spleen constrict and redistribute volume to maintain venous return and cardiac pressures. In fact, 20% to 25% of the total blood volume can be lost without altering circulatory function and pressures.2 In cases of severely reduced CO such as heart failure, the central nervous system (CNS) elicits a sympathetic stimulation that increases vasoconstriction. As the heart fails, this compensatory mechanism reduces blood flow to the liver, kidneys, and other body areas to maintain perfusion to the most vital organs (heart and brain). Because CO, like most other hemodynamic measurements, varies with body size, cardiac index (CI) is often used to describe flow output. CI is obtained by dividing CO by body surface area (BSA) and is reported as liters per minute per square meter (L/minute/m2). BSA is calculated using the patient’s weight and height and the DuBois nomogram (Fig. 16-1). The advantage of using an index is that values are standardized and comparisons can be made among patients of different heights and weights. A commonly cited resting CI reference range for patients of all ages is 2.5 to 4.0 L/minute/m2, with the average for adults being about 3.0 L/minute/m2 (Table 16-1). The CI is highest at 10 years of age and decreases with age to approximately 2.4 L/minute/m2 at age 80 years.2 TABLE 16-1 Hemodynamic Variables, Example Reference Ranges, and Formulas3,4 BP, blood pressure; BSA, body surface area; CVP, central venous pressure (mm Hg); HR, heart rate; MAP, mean arterial pressure (mm Hg); MPAP, mean pulmonary artery pressure (mm Hg); PAWP, pulmonary artery occlusion pressure (mm Hg). ∗Commonly cited references ranges for adults. Sources vary somewhat in the ranges reported. †Conversion factor to convert L/mm Hg to kg/min/m2; 0.0144 is used by some sources.4 ‡Conversion factor to convert mL/mm Hg to g/min/m2; 0.144 is used by some sources.4 An alternative version of the formula includes subtraction of the filling pressures: LVSWI = SV × (MAP − PAWP) × 0.0136; RVSWI = SV × (MPAP − CVP) × 0.0136.2,3 The performance ability of the heart (CO) is determined by both HR and SV. SV is determined by three factors: preload, afterload, and contractility (Fig. 16-2). Figure 16-3 shows ventricular function curves (often called Starling curves) for the right and left ventricles. The horizontal axis represents the volume (preload), and the vertical axis is a measure of the heart’s output: CO, CI, SV, stroke index, or ventricular SWI. Increasing the volume increases output. However, when the pump becomes overstretched, it can no longer eject all of its blood efficiently and CO begins to fall. Continuously measured EDV is the ideal but time-consuming way to assess preload. Most critical care units only measure ventricular volumes on a periodic basis using echocardiography or radionuclide imaging. Therefore, atrial pressures, which can be measured continuously, are used to reflect EDV. During diastole, the atrioventricular valves (tricuspid and mitral) are open. If there is no narrowing or dysfunction of the valves, the pressures in the atrium and ventricle should be the same at end-diastole. The filling pressure for the right heart is right atrial pressure, commonly measured as the central venous pressure (CVP). The filling pressure for the left heart is left atrial pressure, commonly measured as pulmonary artery wedge pressure (PAWP). How these pressures are measured is discussed in Chapter 15. Because a nonlinear relationship exists between the EDV and EDP, filling pressure does not always reflect ventricular volume in the critically ill patient when ventricular compliance is altered. An example in which EDP does not accurately reflect EDV is in patients with increased ventricle chamber stiffness (e.g., myocardial infarction). In these cases, EDP may remain constant as EDV decreases. It is important to understand that pressure is the result of the volume, space, and compliance of the chamber the volume is entering. Forcing 100 mL of water into a small, rigid chamber takes more pressure than filling a compliant balloon with 100 mL of water. Figure 16-4 shows how ventricular pressure is affected by changes in volume and ventricular compliance (distensibility). When compliance is reduced, a much higher pressure is generated for a given volume. Pressure also increases more rapidly as the ventricle fills; thus, the ends of the curves rise more abruptly as the ventricle becomes full and tension is developed in the ventricular walls. • Myocardial ischemia and infarction • Hemorrhagic and septic shock • Right ventricular dilation and overload (causing the septum to shift to the left and impinge on the left ventricle) • Positive end-expiratory pressure (PEEP) or continuous positive airway pressure (CPAP) • Inotropic drugs (increase the strength of myocardial contraction) Factors that increase ventricular compliance include the following: The three main factors affecting the amount of blood returned to the heart are the following: To construct a ventricular function curve, CI, CO, SV, or another measure of heart output is plotted on the vertical axis. Filling pressure (usually pulmonary artery diastolic or PAWP) is plotted on the horizontal axis. A baseline CO measurement is obtained, and the point corresponding to the CO reading and simultaneous pressure reading is plotted (Fig. 16-5A). A fluid challenge is administered, and another set of output and pressure measurements is obtained. Pressure is again plotted against output. As the plotting continues, a Starling (or ventricular function) curve is created. When satisfactory CO is achieved or when CO begins to decline as the filling pressure increases, the fluid challenge is stopped. The pressure that corresponds to the highest CO reading obtained is used to indicate optimal preload. Volume can then be administered as needed to maintain this optimal pressure. It is important to remember that the venous system will begin to “relax” and expand as the volume status is corrected, so it is necessary to follow the patient carefully and reassess the need for additional volume. Positive expiratory pressures, including auto-PEEP (intrinsic PEEP), exaggerate the inspiratory effects of positive-pressure ventilation and maintain increased intrapleural pressure throughout expiration, thus having an even greater potential for decreasing venous return. It has been shown that venous return is affected most by continuous mechanical ventilation (CMV) with PEEP. However, if intravascular volume is maintained or increased to offset the ventilator-induced reduction in venous return, CO may not be affected when positive airway pressure is used. The effect of mechanical ventilation on vascular pressure measurements is discussed in Chapter 15. The peripheral component of afterload is determined by the following: • Elasticity (compliance) of the vessels • Size (radius) of the vessels • Driving pressure (changes in pressure from one end of the vessel to the other) Afterload also increases as the viscosity of the blood increases. This is an important consideration in some patients with chronic pulmonary disease because the concentration of red blood cells often rises above normal in order to increase their oxygen-carrying capacity (see Chapter 7). When hematocrit levels exceed 60%, CO often decreases. Conversely, one of the causes of high CO (hyperdynamic state) is anemia (low hematocrit).
Cardiac Output Measurement
Cardiac Output
Venous Return
Measures of Cardiac Output and Pump Function
Cardiac Index
Variable
Example Reference Range∗
Formula
Cardiac output (CO)
4-8 L/min
CO = direct measurement
Cardiac index (CI)
2.5-4.0 L/min/m2
CI = CO/BSA
Stroke volume (SV)
60-130 mL/beat
SV = CO/HR or EDV − ESV
Stroke volume index (SVI)
30-50 mL/m2
SVI = CI/HR or SV/BSA
Ejection fraction (EF)
65%-75%
EF = SV/EDV or direct measurement
End-diastolic volume (EDV)
120-180 mL/beat
EDV = direct measurement
End-systolic volume (ESV)
50-60 mL
ESV = direct measurement
Rate-pressure product (RPP)
<12,000 mm Hg
RPP = systolic BP × HR
Coronary perfusion pressure (CPP)
60-80 mm Hg
CPP = diastolic BP − PAWP
Left cardiac work index (LCWI)
3.4-4.2 kg/min/m2
LCWI = CI × MAP × 0.0136†
Right cardiac work index (RCWI)
0.4-0.66 kg/min/m2
RCWI = CI × MPAP × 0.0136†
Left ventricular stroke work index (LVSWI)
50-60 g/min/m2/beat
LVSWI = SI × MAP × 0.0136†
Right ventricular stroke work index (RVSWI)
7.9-9.7 g/min/ m2/beat
RVSWI = SI × MPAP × 0.0136‡
Pulmonary vascular resistance (PVR)
<2 units
110-250 dynes-sec/cm5
PVR = (MPAP − PAWP)/CO
PVR = (MPAP − PAWP)/CO × 80§
Pulmonary vascular resistance index (PVRI)
225-315 dynes-sec/cm5/m2
PVRI = (MPAP − PAWP)/CI × 80§
Systemic vascular resistance (SVR)
15-20 units
900-1400 dynes-sec/cm5
SVR = (MAP − CVP)/CO × 80
Systemic vascular resistance index (SVRI)
1970-2400 dynes-sec/cm5/m2
SVRI = (MAP − CVP)/CI × 80§
Determinants of Pump Function
Preload
Ventricular Function Curves
Ventricular Compliance
Factors that Affect Venous Return, Preload, and Cardiac Output
Clinical Applications of Ventricular Function Curves
Effects of Mechanical Ventilation on Preload and Venous Return
PEEP and CPAP
Afterload
Peripheral Resistance
Increased Vascular Resistance