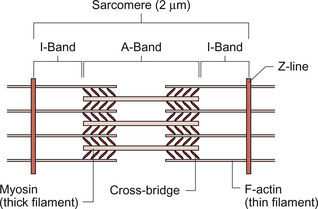
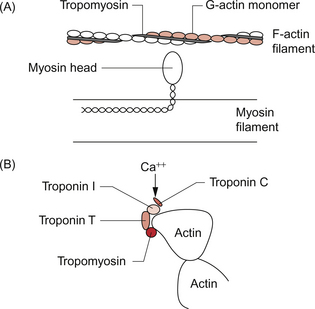
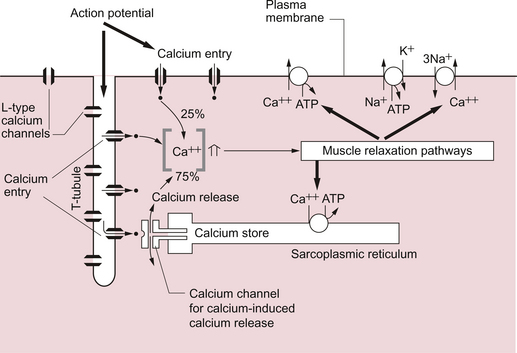
2 In skeletal muscle, fibres can be activated individually or in groups to vary the strength of the contraction. In the heart, the coordinated contraction resulting from the spread of activity across the atria and the ventricles requires that the cardiac muscle cells are electrically connected through gap junctions. In skeletal muscle the strength of contraction of individual fibres can be varied by changing the frequency of action potentials, but this is not an option for the heart where rhythmic contractions involve all of the cardiac muscle cells. Instead, the strength of contraction of cardiac muscle is regulated, as in smooth muscle, by varying the intracellular calcium concentration during activation of the cells. This provides a target site for drugs which affect the strength of cardiac contraction and hence cardiac output. The actions of these ‘inotrope’ drugs are discussed in Chapter 4. The fundamental contractile unit in both skeletal and cardiac muscle is the sarcomere (Fig. 2.1). These units are about 2 μm long and are defined at each end by the Z line which is formed from the protein α-actinin. Attached to the Z lines are the thin filaments made from F-actin which in turn consist of G-actin monomers joined together, in a structure sometimes said to resemble a helical string of beads, to form the thin filament (Fig. 2.2). These actin thin filaments are arranged in a parallel sandwich structure with the protein myosin (thick filament). Under polarized light microscopy the arrangement of the actin and myosin protein creates a striated (striped) appearance in which the A band is generated by the myosin filaments and the I band is composed mainly of actin (Fig. 2.1). Parallel bundles of sarcomeres are joined end to end to make up a myofibril. The myocytes primarily contain bundles of myofibrils together with mitochondria and the cell nucleus, which is displaced to one side of the cell. Unlike skeletal muscle the myocytes of the atria and ventricles are not attached to specific skeletal insertion points by tendons but instead they are joined together in a branched meshwork to form a muscular bag in which the cells contract against their attachment to adjacent cells. Each individual myocyte in the adult human heart is about 50–100 μm in length and about 10–20 μm in diameter. In order to work as an effective contractile unit and to permit electrical activity to spread across heart muscle the individual muscle cells must be both physically joined together and electrically connected. This is achieved by the presence of ‘intercalated discs’—sites of apposition and thickening of the sarcolemma of adjacent cells. The intercalated discs contain both high conductance gap junctions (connexons) which provide for electrical continuity and desmosomes containing the protein cadherin to form a junction with adequate physical strength. An increase in intracellular [Ca++] causes contraction of the myocardial cell by a sliding filament mechanism similar to that in skeletal muscle. The actin and myosin filaments pass over each other as a result of the breaking and reforming of cross-bridges between the filaments (Fig. 2.2). The cross-bridges are formed between the heads of the myosin-filaments and the actin filaments. When the [Ca++i] (the intracellular calcium ion concentration) rises the calcium ions bind to troponin C, a part of the three protein troponin complex. Troponin C is attached to tropomyosin, a protein which in the resting state shields a specific myosin binding region on the actin filament. The resulting ‘cross-bridge’ between myosin and actin undergoes a structural change which moves the actin filament over the myosin filament producing a small contraction. The myosin head then disengages and the process is repeated causing the actin to ‘walk’ down the myosin filament. The force of contraction depends on the number of cross-bridges formed, a parameter which in turn depends on the [Ca++] inside the muscle cell. Under resting conditions only a relatively small proportion of the potential total cross-bridge formation actually occurs. This means that physiological stimulation, via sympathetic nervous system activation, and drugs which increase intracellular [Ca++] can generate a more forceful cardiac muscle contraction than occurs at resting levels. Each cycle of cross-bridge formation involves the hydrolysis of an ATP molecule to alter the configuration of the myosin head as part of the contraction process. Cardiac muscle cells are continually contracting and require substantial amounts of energy. Metabolically they are similar to ‘slow’ skeletal muscle fibres in that they derive their energy from ATP generated by oxidative phosphorylation and the myocytes thus contain large numbers of mitochondria. It must be appreciated that, like any muscle, the contraction of cardiac muscle, particularly in the left ventricle, impedes the flow of blood through the coronary blood vessels and thus the heart muscle is only effectively perfused during its relaxation (diastolic) phase (see Chapter 5). A period of powerful cardiac contractions at a rapid rate such as might occur during exercise may result in the oxygen supply to the myocardium being insufficient to meet the metabolic demand. Responses to exercise are discussed in Chapter 13. As previously noted, the force of cardiac muscle contraction depends on the intracellular [Ca++]. Opposite each Z line there is a tubular structure, the T tubule, running at right angles to the plasma membrane of the cell (Fig. 2.3). The T tubules help to spread electrical excitation rapidly into the cell and they run close to the sarcoplasmic reticulum (SR) in which Ca++ ions are stored. Ca++ is pumped into the stores by using Ca++ ATPase pumps which are regulated by the inhibitory protein phospholamban. The Ca++ used to trigger contraction of cardiac muscle therefore comes from two sources, the SR (about 75% of the total) and also transmembrane flux of Ca++ from the extracellular fluid (about 25% of the total). This is in contrast to skeletal muscle which only uses SR stores of Ca++ for contraction. The resting intracellular [Ca++] is about 0.1 μmol/L and when an action potential (see p. 21) occurs in a cardiac muscle cell it triggers an initial increase in the intracellular calcium ion [Ca++i] concentration. The action potential results in an inward flow of calcium from the extracellular fluid where the ionized calcium concentration is about 1.2 mmol/L. This takes place through L-type calcium channels located in the T tubules and in the plasma membrane. The initial small increase in [Ca++i] causes the release of further calcium ions from the SR stores—the so-called calcium-induced calcium release. This is mediated by a Ca++ binding site on the SR which is part of a calcium channel protein often referred to as a ‘ryanodine-sensitive receptor’ or as a ‘foot protein’ (Fig. 2.3). As a result of calcium release from the SR the [Ca++i] increases, normally to about 0.5–2 μmol/L. In heart failure (see Chapter 6) there are significant alterations in how myocyte [Ca++] is regulated. During relaxation some Ca++ has to be exported back out of the cell and some replaced into the SR. Ca++ is predominantly expelled from the myocyte via a 3Na+−Ca++ exchanger which uses the inward ‘downhill’ movement of the 3Na+ to move Ca++ out of the cell (Fig. 2.3). This mechanism per se does not consume ATP although the Na+/K+-ATPase actively expels Na+ across the plasma membrane in order to maintain the electrochemical gradient for Na+. A portion of the Ca++ is actively expelled from the cell across the plasma membrane by Ca ATPases. ATP is also used to pump Ca++ back into the SR stores. Within the SR much of the calcium is stored as ionized Ca++. However some is attached to calcium binding proteins of which calsequestrin is one of the most important. Physiological stimulation of sympathetic nerves to the heart results in an increased force of contraction (see Chapter 4). The β1-adrenoceptor activation leads to a rise in intracellular cyclic AMP (see Fig. 4.6), a second messenger which activates several protein kinases. Subsequent phosphorylation of the protein phospholamban accelerates transport of Ca++ into the SR thus favouring retention of Ca++ in the SR at the expense of efflux back across the plasma membrane. Contractility of the heart is therefore increased by raising the amount of Ca++ stored in the SR. The rate of relaxation of cardiac muscle is also increased as the Ca++ re-enters the SR more quickly. The effects of cAMP in these events can be manipulated by drugs such as milrinone and caffeine which act as phosphodiesterase inhibitors and hence prolong the half-life of cAMP. The resting potential of a cardiac muscle cell is about −85 mV and, as in other excitable cells, this occurs as a result of the ionic concentration gradients maintained by the action of the Na+/K+-ATPase (see Chapter 1). The intracellular [K+i] is about 140 mmol/L whilst the extracellular [K+o] is about 4 mmol/L. We can consider a theoretical cell with such a concentration gradient for K+ and, initially, an equal number of positive and negative charges inside the cell. There is a diffusion gradient for positively charged potassium ions to move out of this theoretical cell and thus create a charge imbalance (potential difference) across the cell membrane with the inside of the cell negatively charged. The negative charge inside cells is mainly in the form of organic phosphates and ionizable groups on proteins, molecules which are too large to follow the K+ across the cell membrane. Eventually a situation is reached where the tendency for K+ ions to move out of the cell down the concentration gradient is balanced by the electrical gradient which will tend to move K+ ions back into the cell. This concept of the balance between the diffusive gradient and the electrical gradient is the basis for the derivation of the Nernst equation.
CARDIAC MUSCLE STRUCTURE AND FUNCTION
Cardiac muscle
Structure of cardiac muscle
Contractile mechanism in cardiac muscle
Regulation of intracellular [Ca++] in cardiac muscle
Cardiac electrical activity
Resting potential of ventricular muscle cells
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


CARDIAC MUSCLE STRUCTURE AND FUNCTION