15
Cardiac Masses and Potential Cardiac Source of Embolus
Basic Principles
The first step in assessing a possible cardiac mass is to ensure that the echocardiographic findings represent an actual mass rather than an ultrasound artifact. As discussed in detail in Chapter 1, artifacts can be caused by electrical interference, characteristics of the ultrasound transducer/system, or various physical factors influencing image formation from the reflected ultrasound signals. These include beam-width artifact, near-field “ring-down,” and multipath artifact. Appropriate transducer selection, scanning technique, and evaluation from multiple examining windows will help to distinguish artifacts from actual anatomic structures.
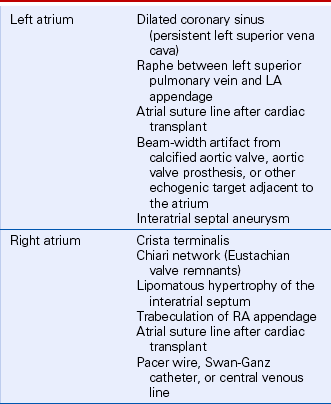
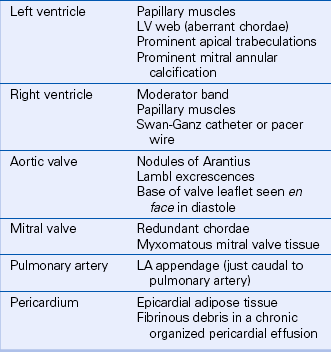
Besides ultrasound artifacts, several normal structures and normal variants may be mistaken for a cardiac mass (Table 15-1). In the ventricles, normal trabeculae, aberrant trabeculae or chordae (ventricular “webs” or false tendons) (Fig. 15-1), muscle bundles (such as the moderator band), or the papillary muscles may be mistaken for abnormal structures.
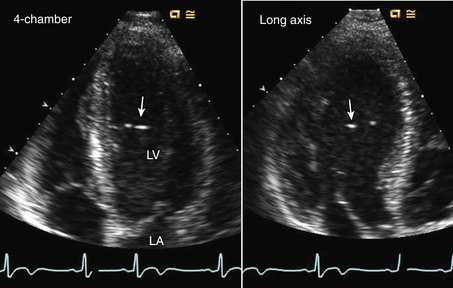
Figure 15–1 ![]() LV web.
LV web.
Aberrant trabeculation or “web” seen in an apical four-chamber view (left) and long-axis view (right) appearing as bright echoes in the LV chamber with a thin structure traversing the LV chamber seen on video images.
Valve anatomy includes a wide range of normal variation, and the appearance of a normal (but often unrecognized) structure, such as a nodule of Arantius, on the aortic valve may be considered incorrectly to represent a cardiac mass. The belly of a valve leaflet, if cut tangentially, may appear as a “mass” when it actually is a portion of the leaflet itself seen en face. In the atrium, normal ridges adjacent to the venous entry sites (Figs. 15-2 and 15-3), normal trabeculations (Fig. 15-4), postoperative changes (see Fig. 9-31), and distortion of the free wall contour by structures adjacent to the atrium (Fig. 15-5) all may be diagnosed erroneously as a cardiac mass.
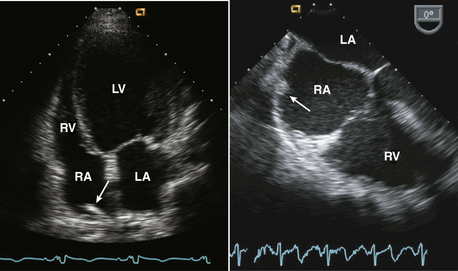
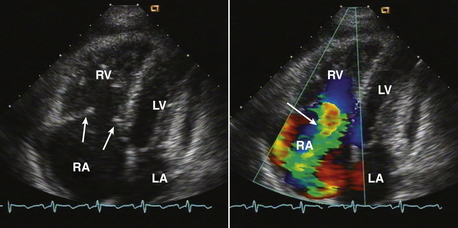
Figure 15–2 Christa terminalis.
Normal appearance (arrow) in the RA in a TTE apical four-chamber view (left) and a TEE view (right).
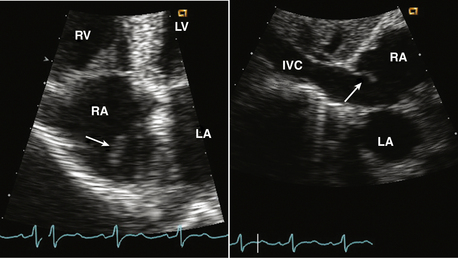
Figure 15–3 Eustachian valve.
Prominent valve at the entrance of the inferior vena cava into the RA seen in an apical four-chamber view (left) might be mistaken for a cardiac mass. A subcostal view (right) shows the inferior vena cava and valve more clearly.
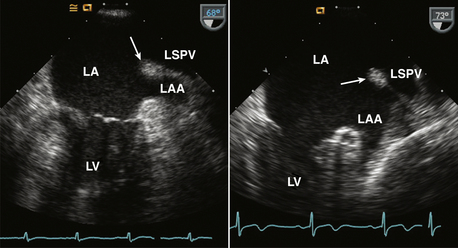
Figure 15–4 ![]() Ridge between the LA appendage (LAA) and left superior pulmonary vein (LSPV).
Ridge between the LA appendage (LAA) and left superior pulmonary vein (LSPV).
TEE views of this normal ridge in two different patients are shown. Sometimes this ridge is evident on TTE parasternal short-axis or apical two-chamber views.
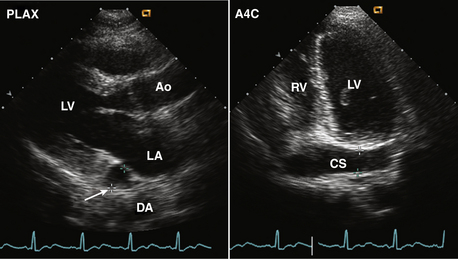
Figure 15–5 ![]() Persistent left superior vena cava.
Persistent left superior vena cava.
Diagnosis is based on the finding of a dilated coronary sinus (CS) posterior to the LA (arrow) seen in a parasternal long-axis view (left). If there is ultrasound “dropout” from the wall of the coronary sinus, the abnormal contour of the LA may be mistaken for a mass. In the posteriorly angulated apical four-chamber view, the dilated coronary sinus is seen (right), which can be demonstrated to be connected to the RA by angulation back to a four-chamber view.
Definitive diagnosis of an intracardiac mass by echocardiography is based on:
 Excellent image quality, which may require the use of a high-frequency (5- or 7.5-MHz), short-focus transducer to evaluate the LV apex from the transthoracic (TTE) approach and the use of transesophageal (TEE) imaging to evaluate posterior cardiac structures (e.g., LA, mitral valve). Three-dimensional (3D) echocardiography may better define the location and geometry of the mass.
Excellent image quality, which may require the use of a high-frequency (5- or 7.5-MHz), short-focus transducer to evaluate the LV apex from the transthoracic (TTE) approach and the use of transesophageal (TEE) imaging to evaluate posterior cardiac structures (e.g., LA, mitral valve). Three-dimensional (3D) echocardiography may better define the location and geometry of the mass.
 Identification of the mass throughout the cardiac cycle, in the same anatomic region of the heart, from more than one acoustic window. This decreases the likelihood of an ultrasound artifact.
Identification of the mass throughout the cardiac cycle, in the same anatomic region of the heart, from more than one acoustic window. This decreases the likelihood of an ultrasound artifact.
 Knowledge of the normal structures, normal variants, and postoperative changes that may simulate a cardiac mass.
Knowledge of the normal structures, normal variants, and postoperative changes that may simulate a cardiac mass.
 Integration of other echocardiographic findings (e.g., rheumatic mitral stenosis and LA enlargement in a patient with suspected LA thrombus) and clinical data in the final echocardiographic interpretation.
Integration of other echocardiographic findings (e.g., rheumatic mitral stenosis and LA enlargement in a patient with suspected LA thrombus) and clinical data in the final echocardiographic interpretation.
Infectious Cardiac Masses
Infectious cardiac masses include valvular vegetations, which are seen in patients with endocarditis (bacterial or fungal). Noninfectious vegetations also occur in patients with nonbacterial thrombotic endocarditis (NBTE, or marantic endocarditis). Vegetations typically are irregularly shaped, attached to the upstream side of the valve leaflet (e.g., LA side of the mitral valve, LV side of the aortic valve), and exhibit chaotic motion that differs from that of the leaflets themselves (Fig. 15-6). Valvular regurgitation is a frequent but not invariable accompaniment of endocarditis. Valvular stenosis due to the vegetation is rare. Paravalvular abscess, which also presents as a cardiac mass, may be difficult to recognize on TTE imaging but can be diagnosed with a high sensitivity and specificity on TEE echocardiography. Infectious cardiac masses are discussed in detail in Chapter 14.
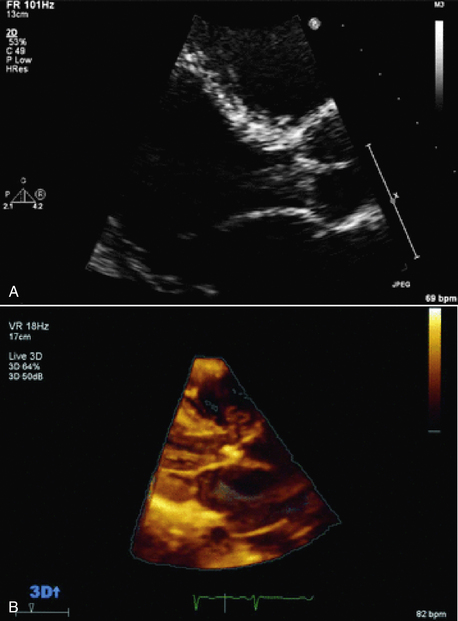
Figure 15–6 2D and 3D images of an aortic valve vegetation.
In both the standard TTE 2D long-axis view (A) and with real-time TTE 3D imaging (B), a mass is seen prolapsing from the aortic valve into the LV outflow tract. (From Zaragoza-Macias E, Chen MA, Gill EA: Real time three-dimensional echocardiography evaluation of intracardiac masses. Echocardiography 29[2]:207-219, 2012.)
Cardiac Tumors
Nonprimary cardiac tumors are approximately 20 times more common than primary cardiac tumors. Tumors can involve the heart by direct invasion from adjacent malignancies (lung, breast), by lymphatic spread, or by metastatic spread of distant disease (lymphoma, melanoma) (Fig. 15-7). In an autopsy series of patients with a malignancy, cardiac involvement is present in approximately 10% of cases, although clinical recognition of cardiac involvement occurs less frequently. Melanoma has the highest rate of pericardial metastases, but because there are relatively few patients with melanoma, a cardiac tumor is more likely to represent a more prevalent malignancy, as shown in Table 15-2.
TABLE 15-2
Origin of Metastatic Cardiac Tumors in Adults (In Order of Frequency)
Lung
Lymphoma
Breast
Leukemia
Stomach
Melanoma
Liver
Colon
Data from Abraham KP, Reddy V, Gattuso P: Neoplasms metastatic to the heart: Review of 3314 consecutive autopsies. Am J Cardiovasc Pathol 3:195-198, 1990.
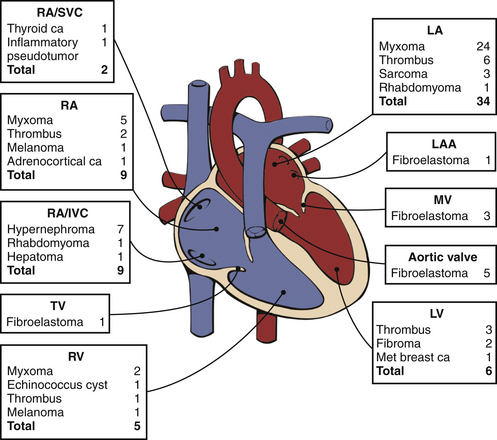
Figure 15–7 Distribution and pathologic characteristics of cardiac masses according to intracardiac attachment site.
In this series of 75 consecutive patients undergoing surgery for cardiac mass removal, masses were most often seen in the LA (46%), followed by the RA/IVC/SVC (27%), LV (8%), and RV (7%), plus 12% were attached to a valve. The most common causes of masses requiring excision were myxomas (41%), thrombi (16%), fibroelastoma (13%), and hypernephroma (9%). The baseline or postprocedure intraoperative TEE altered management in 16% of cases. ca, carcinoma; IVC, inferior vena cava; LAA, LA appendage; MET, metastatic; MV, mitral valve; SVC, superior vena cava; TV, tricuspid valve. (From Dujardin KS, Click RL, Oh JK: The role of intraoperative transesophageal echocardiography in patients undergoing cardiac mass removal. J Am Soc Echocardiogr 13:1080-1083, 2000.)
Nonprimary cardiac tumors can affect the heart by:
 Invasion of the pericardium, epicardium, myocardium, or endocardium
Invasion of the pericardium, epicardium, myocardium, or endocardium
 Production of biologically active substances
Production of biologically active substances
 Toxic effects of treatment on the heart (e.g., radiation or chemotherapy)
Toxic effects of treatment on the heart (e.g., radiation or chemotherapy)
Cardiac malignancies most often involve the pericardium and epicardium (approximately 75% of metastatic cardiac disease), presenting as a pericardial effusion, with or without tamponade physiology (Fig. 15-8). Because echocardiographic diagnosis of the cause of a pericardial effusion rarely is possible, the diagnosis of a pericardial effusion (and particularly tamponade) in a patient with a known malignancy should alert the clinician to the possibility of cardiac involvement. Confirmation of the diagnosis requires examination of pericardial fluid and, if necessary, pericardial biopsy. The differential diagnosis of a pericardial effusion in a patient with a known malignancy includes radiation pericarditis and idiopathic pericarditis (which is common in patients with cancer), in addition to metastatic disease. Repeat echocardiographic evaluation of patients with a malignant pericardial effusion often is needed after the initial diagnosis for assessment of therapeutic interventions and follow-up for recurrent effusion.
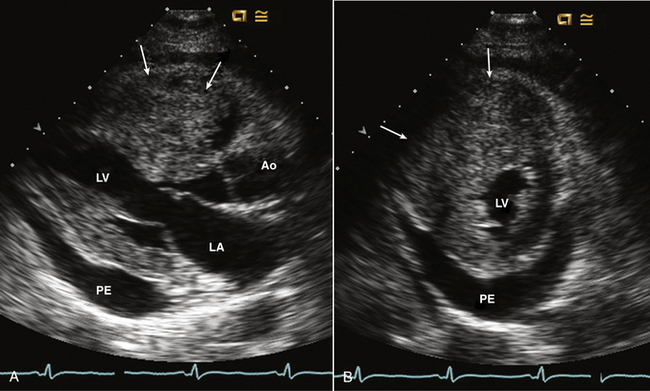
Figure 15–8 Metastatic hepatocellular carcinoma.
A mass is seen involving the myocardium with marked thickening of the RV free wall and near cavity obliteration in parasternal long-axis (A) and short-axis (B) views. A pericardial effusion (PE) also is present.
A specific type of cardiac involvement by tumor that should be recognized by the echocardiographer is the extension of renal cell carcinoma up the inferior vena cava (Fig. 15-9). A “fingerlike” projection of a tumor may protrude into the right atrium (RA) from the inferior vena cava, and the tumor can be followed retrograde (from a subcostal approach) back to the kidney. Correlation with other wide-view imaging techniques is needed for full delineation of the tumor extent. Uterine tumors also occasionally present in this fashion.
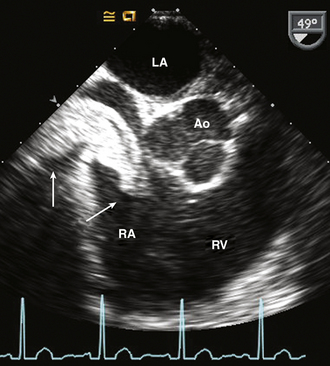
Figure 15–9 Renal cell carcinoma.
TEE imaging in a patient with renal cell carcinoma showing the extension into the RA from the inferior vena cava without involvement of the atrial wall, septum, or valves.
Tumors also can affect the cardiac structures indirectly, as is seen in carcinoid heart disease (Fig. 15-10). Metastatic carcinoid tissue in the liver produces biologically active substances, including serotonin, which cause abnormalities of the right-sided cardiac valves and endocardium. Typical changes include thickening, retraction, and increased rigidity of the tricuspid and pulmonic valve leaflets, resulting in valvular regurgitation or, less often, valvular stenosis. Left-sided valvular involvement is rarely seen, possibly because of a lower concentration of the active molecules after passage through the lungs. While metastatic carcinoid disease is rare, the echocardiographic findings are pathognomonic and may lead to the diagnosis in a patient in whom it was not considered previously. About one third of patients with carcinoid tumors have cardiac involvement, and half the deaths in carcinoid patients are due to heart failure resulting from severe tricuspid regurgitation.
Primary
As for tumors elsewhere in the body, the distinction between benign and malignant primary cardiac tumors is based on pathologic examination of tissue and its tendency to invade adjacent tissue or metastasize to distant sites (Table 15-3). Although 75% of primary cardiac tumors are benign, a pathologically benign cardiac tumor can have “malignant” hemodynamic consequences if it obstructs the normal pattern of blood flow. Thus, the echocardiographic examination includes definition of both the anatomic extent of a cardiac tumor and its physiologic consequences.
TABLE 15-3
Primary Cardiac Tumors in Adults
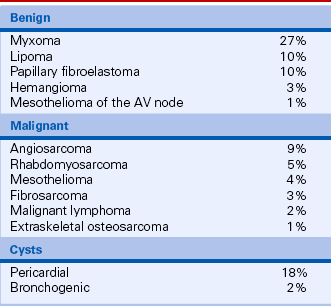
Data from McAllister HA, Fenoglio JJ: Tumors of the cardiovascular system. In Atlas of Tumor Pathology, Fasicle 15, 2nd Series. Bethesda, MD: Armed Forces Institute of Pathology, 1978.
Benign Primary Cardiac Tumors
Myxomas account for 27% of primary cardiac tumors. Cardiac myxomas most often are single, arising from the fossa ovalis of the interatrial septum and protruding into the LA (in approximately 75% of cases) (Fig. 15-11). Other sites of origin include the RA (18%), the LV (4%), and the right ventricle (RV) (4%). More than one site can occur in an individual patient (5% of cases).
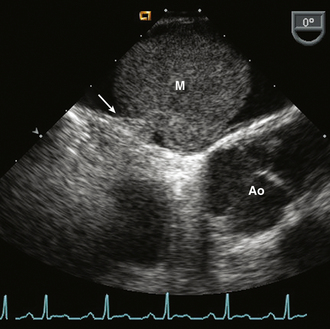
Figure 15–11 ![]() LA myxoma (M).
LA myxoma (M).
A mass arising from a thin stalk (arrow) attached to the fossa ovalis of the interatrial septum seen in a TEE view.
The clinical presentation of a cardiac myxoma can include constitutional symptoms (fever, malaise), clinically evident embolic events, and symptoms of mitral valve obstruction. A myxoma also may be an unexpected finding on a study requested for other clinical indications.
An LA myxoma may nearly fill the LA chamber (Fig. 15-12), with prolapse of the tumor mass across the mitral annulus into the LV in diastole (accounting for the tumor “plop” on auscultation). The mass often has an irregular shape characterized by protruding “fronds” of tissue or a “grape cluster” appearance. The echogenicity of the mass may be nonhomogeneous, and sometimes areas of calcification are noted.
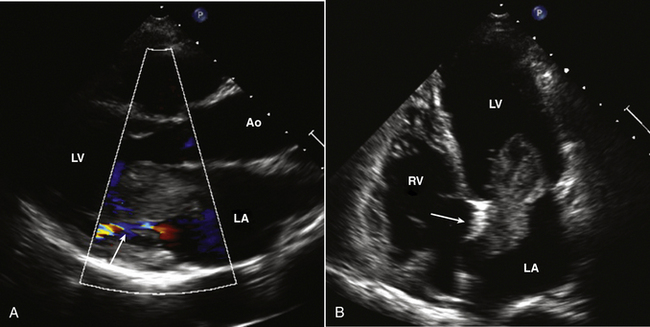
Figure 15–12 Large LA myxoma.
A parasternal long-axis view (A) shows the tumor prolapsing into the mitral orifice in diastole, which results in functional mitral stenosis with only a narrow flow stream seen with color Doppler. In the apical view (B), the attachment to the septum (arrow) and the inhomogeneous echogenicity of the myxoma are seen.
 To identify the site of tumor attachment
To identify the site of tumor attachment
 To ensure that the tumor does not involve the valve leaflets themselves
To ensure that the tumor does not involve the valve leaflets themselves
Postoperatively, complete excision should be documented by echocardiography. Sequential long-term follow-up is indicated, because recurrent myxomas have been reported, particularly with a familial form of this disease, with multiple myxomas, or with a less than full-thickness excision.
A papillary fibroelastoma is a benign cardiac tumor that arises on valvular tissue, thus mimicking the appearance of a valvular vegetation. A papillary fibroelastoma appears as a small mass attached to the aortic or mitral valve with motion independent from the normal valve structures (Fig. 15-13). A papillary fibroelastoma also may be seen attached to the tricuspid or pulmonic valve or at nonvalvular sites. Unlike a vegetation, a fibroelastoma is more often found on the downstream side of the valve (LV side of mitral valve, aortic side of aortic valve). The histologic appearance is very similar to the smaller Lambl excrescences, which can be seen on normal valves in the elderly. Usually a small papillary fibroelastoma is of no clinical significance; the relationship of larger benign valve tumors to embolic events is controversial. In addition, some cases of superimposed thrombus formation resulting in systemic embolic events have been described. Often these tumors are better visualized on TEE imaging.
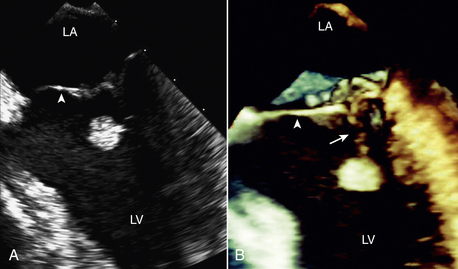
Figure 15–13 Papillary fibroelastoma on 2D and 3D imaging.
A 49-year-old man experienced recurrent transient ischemic attacks. 2D (A) and 3D (B) TEE clearly show an approximately 1-cm diameter papillary fibroelastoma attached by a stalk (arrow) to the chordal apparatus of the ventricular aspect of the mitral valve (arrowhead). The mass was highly mobile, and the surface of the tumor had a “shimmering” edge when imaged in real time. There were no other abnormalities of the mitral valve, and there was no mitral regurgitation. The tumor was resected using a minimally invasive, robotic approach. (From Bruce CJ: Cardiac tumours: Diagnosis and management. Heart 97[2]:151-60, 2011.)
Lipomatous hypertrophy of the interatrial septum presents as a cardiac mass that may be mistaken for a tumor. Lipomatous hypertrophy typically involves the superior and inferior fatty portions of the atrial septum, sparing the fossa ovalis region (Fig. 15-14). However, symmetric ellipsoid-shaped enlargements of the interatrial septum also have been described. If the cause of atrial septal hypertrophy is unclear on echocardiography, CT scanning may establish the diagnosis of lipomatous hypertrophy by showing the characteristic radiographic density of adipose tissue.
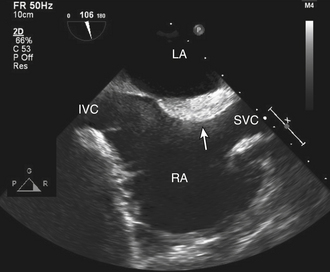
Figure 15–14 ![]() Lipomatous hypertrophy (arrow)
Lipomatous hypertrophy (arrow)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree