Chapter 10
Cardiac Magnetic Resonance Imaging
1. How does cardiac magnetic resonance imaging (CMR) produce images?
Similar to echocardiography, CMR allows the generation of images of the heart without exposure to ionizing radiation. Although the spatial resolution of CMR is comparable to echocardiography (approximately 1 mm), the contrast-to-noise and signal-to-noise ratios are far superior (Fig. 10-1). The latter allows easier delineation of borders between tissues and between blood pool and tissue. The contrast between blood pool and myocardium (see Fig. 10-1) is generated using differences in signal properties of the different tissues without the use of contrast agents. CMR is also not limited by “acoustic windows” that may hinder echocardiography, and images can be obtained in any tomographic plane. Finally, CMR can provide information about tissue characteristics using differences in T1 and T2 signals, and with addition of contrast agents.
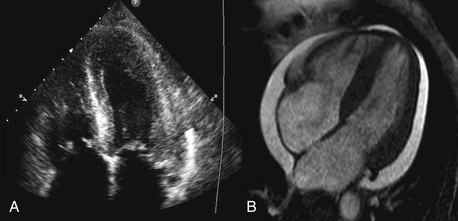
Figure 10-1 Comparison of (A) an echocardiographic and (B) cardiac MRI (CMR) cine image of a four-chamber view of the same patient. The superior contrast-to-noise and signal-to-noise ratios are clearly evident in the CMR image, with clear delineation of the endocardial and epicardial borders of both ventricles.
3. What are the limitations of CMR?
The major limitation of CMR is availability. Given the cost, the special construction necessary to host a CMR system, and the technical expertise and support necessary, CMR is not widely available at all centers. CMR is limited with respect to portability, unlike echocardiography where imaging can be performed at the patient’s bedside. There is also a set of contraindications (listed later) that limits the use of this technology in selected patient populations. Image acquisition can be challenging in patients with an irregular cardiac rhythm or difficulty holding their breath, though newer real-time techniques provide an opportunity to overcome these barriers (Fig. 10-2). Finally, because patients have to lie still in a long hollow tube for up to an hour during imaging, claustrophobia may become an important limiting factor.
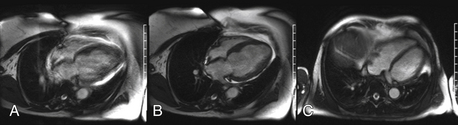
Figure 10-2 A, Four-chamber view in a patient with difficulty holding their breath (blurry). B, The same patient who was able to breath-hold after the acquisition period was shortened. C, Another patient with a real-time acquisition while breathing freely.
4. What are the common imaging pulse sequences used in CMR?
5. What are the appropriate uses of CMR?
6. What is delayed enhancement (DE) CMR imaging?
One of the unique aspects of CMR is the ability to identify myocardial scar and/or fibrosis. This is most commonly performed using DE imaging. Gadolinium-based contrast agents are first administered, then after waiting approximately 10 minutes and allowing time for the contrast to distribute into areas of scar and fibrosis, an inversion recovery sequence is performed. This sequence nulls (makes it black) normal myocardium and anything that is bright within the myocardium is most likely myocardial scar or fibrosis (Fig. 10-3).
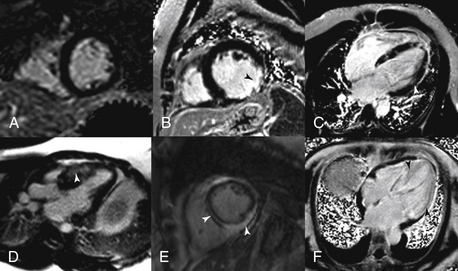
Figure 10-3 Delayed enhancement (DE) patterns. A, A normal short-axis DE image. B, A transmural scar in the circumflex territory. C, Cardiomyopathy secondary to sarcoidosis. D, Hypertrophic obstruction cardiomyopathy with basal anterior septal midmyocardial DE. E, Myocarditis with both midmyocardial and epicardial DE. F, Cardiac amyloidosis with diffuse enhancement of the entire left ventricle.
7. Does CMR have a role in the evaluation of chest pain?
In patients with chest pain syndrome, CMR stress testing can be performed to assess flow-limiting coronary stenosis. This is most appropriate in patients with intermediate pretest probability of CAD with uninterpretable ECG or who are unable to exercise. The two stress methods used are vasodilator perfusion CMR or dobutamine stress function CMR. Vasodilator stress testing is performed identically to nuclear stress testing with the use of adenosine. With peak coronary vasodilation, gadolinium contrast agent is administered and perfusion of the myocardium during the first pass of the contrast agent is captured. Areas of perfusion abnormality can be detected as dark areas (Fig. 10-4). Resting perfusion is also commonly performed, mainly to differentiate imaging artifacts from a true perfusion defect. This is then followed by DE imaging to assess for myocardial scar. Alternatively, graded doses of dobutamine can be administered to provide a sympathetic stress with cine imaging to identify regions of wall motion abnormality, in a manner similar to echocardiography.
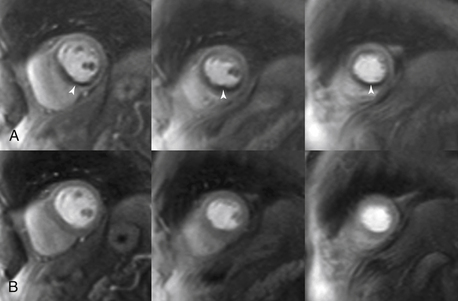
Figure 10-4 Adenosine perfusion imaging. A, Peak stress short-axis and four-chamber perfusion images showing ischemia (arrows) in the basal to apical inferior and inferior septal segments, consistent with ischemia in the right coronary artery territory. B, Rest images illustrate normal perfusion.
8. Can CMR coronary angiography be used to assess chest pain?
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


