WHO 1
Risk no higher than general population
WHO 2
Small increased risk of maternal mortality and morbidity
WHO 3
Significant increased risk of maternal mortality and morbidity. Expert cardiac and obstetric pre-pregnancy, antenatal and postnatal care required
WHO 4
Pregnancy contraindicated: very high risk of maternal mortality or severe morbidity. Termination should be discussed. If pregnancy continues, care as for class 3
Conditions with WHO 1 Pregnancy Risk
Uncomplicated, small or mild:
Pulmonary stenosis
Ventricular septal defect
Patent ductus arteriosus
Mitral valve prolapse with no more than trivial mitral regurgitation
Successfully repaired simple lesions, e.g.:
Ostium secundum atrial septal defect
Ventricular septal defect
Patent ductus arteriosus
Total anomalous pulmonary venous drainage
Isolated ventricular extrasystoles and atrial ectopic beats
Conditions with WHO 2 Pregnancy Risk
Unoperated atrial septal defect
Repaired tetralogy of Fallot
Most arrhythmias
Conditions with WHO 2 or 3 Pregnancy Risk Depending on Individual
Mild left ventricular impairment
Hypertrophic cardiomyopathy
Native or tissue valvular heart disease not considered WHO 4
Marfan syndrome without aortic dilatation
Heart transplantation
Conditions with WHO 3 Pregnancy Risk
Mechanical valve
Systemic right ventricle (e.g. congenitally corrected transposition, simple transposition post Mustard or Senning repair)
Post Fontan operation
Cyanotic heart disease
Other complex congenital heart diseases
Conditions with WHO 4 Pregnancy Risk
Pulmonary arterial hypertension of any cause
Severe systemic ventricular dysfunction
NYHA III–IV or LVEF, <30 %
Previous peripartum cardiomyopathy with any residual impairment of left ventricular function
Severe left heart obstruction
Marfan syndrome with aorta dilated >40 mm
60.3 Contraception
Helping patients prevent unplanned pregnancies and the cardiovascular complications that they might cause is an important consideration in patients of child-bearing age with complex cardiac disease. Some contraceptives carry cardiovascular risks for which patients should be counselled. Medroxyprogesterone should be avoided in those with reduced LV function as it is associated with fluid retention. Contraceptive pills containing estrogen or estradiol are associated with increased risk of thromboembolism and should be avoided in those at pre-existing increased risk. Implanting of intrauterine devices has a 5 % risk of vasovagal episode and should only be performed in a hospital environment in those with complex cardiac disease [10] (Table 60.2).
Table 60.2
Effectiveness of different methods of contraception
Method | % of women experiencing an unintended pregnancy within the first year of use | |
|---|---|---|
Typical use | Perfect use | |
No method | 85 | 85 |
Diaphragm | 16 | 6 |
Condom (female) | 21 | 5 |
Condom (male) | 15 | 2 |
Combined pill and minipill | 8 | 0.3 |
Depo-Provera | 3 | 0.3 |
Mirena – intrauterine device | 0.1 | 0.1 |
Female sterilisation | 0.5 | 0.5 |
Male sterilisation | 0.15 | 0.10 |
60.4 Physiological Changes of Pregnancy
60.4.1 Cardiac Physiological Changes
Pregnancy causes a progressive and increasing cardiovascular challenge, typically ending with the peak stress during labour. Stroke volume increases from week 8 and peaks at week 20. The heart rate increases from week 5 and peaks at week 32 with an increase of 17 % [12]. These factors combine to cause cardiac output to increase by week 5 gestation and peaks at 24–32 weeks with 48 % rise compared to pre-conception levels [12] with further rises of up to 80 % during and after labour (Fig. 60.1). The additional cardiac output is mainly directed towards the kidneys, uterus and skin. By term, the uterus receives 15–20 % of cardiac output. Cardiac output is increased additionally by 15 % in twin pregnancies compared to singleton pregnancies [13]. Cardiac output approximates pre-pregnancy values within 2 weeks of delivery with subtle further resolution completing by 6 months [13]. An increase in LV wall thickness and a 50 % increase in left ventricular mass suggest myocardial hypertrophy; these structural changes resolve more gradually than the functional cardiac changes of pregnancy but are typically resolved by 6 months. The left atrial dimensions increase by 16 %, peaking at 28 weeks [12]. Valve annuli dilate, peaking at 14 % increase in area in weeks 32–38 [12], and hence physiological valve regurgitation increases throughout pregnancy, due to reduced leaflet coaptation, with the majority resolved by 6 weeks postpartum [14]. Pulmonary capillary wedge pressure and pulmonary pressures are unchanged. Myocardial contractility also increases in the first two trimesters [12] (Chap. 54).
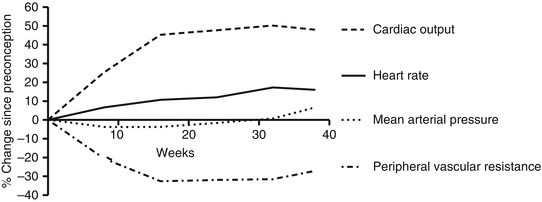

Fig. 60.1
Physiological changes from pre-conception through term (Figure constructed based on the data from Robson et al. [12])
The gravid uterus progressively exerts pressure on the inferior vena cava which can reduce filling pressure. Cardiovascular adaptations can be significantly altered by posture in the second half of pregnancy, and the effect of the gravid uterus restricting venous return whilst lying supine can cause up to 30 % reduction in cardiac output. This can cause supine hypotensive syndrome which can cause faintness, dyspnoea, dizziness, nausea, visual disturbances and numbness and can lead to syncope [15]. Pregnant women experiencing this naturally shift position to displace the compressive uterus, but in situations where the women is restrained or sedated such as surgery, this is an important consideration. The aorta increases in size from week 24 onwards, returning to baseline in the majority of women by 6 weeks postpartum [16]. Arterial compliance increases, by as much as 30 % in the first trimester, and remains elevated through pregnancy [17].
60.4.2 Vascular Physiological Changes During Pregnancy
The plasma volume increases from week 4, reaching up to 30–50 % from baseline early in the third trimester. Systemic vascular resistance is measurably reduced by week 6 and is reduced by 34 % by week 20 [12]. Systemic vasodilation triggers baroreceptors and triggers the renin, aldosterone and angiotensin II levels to rise to maintain circulating volumes and pressures by retaining water and sodium. Aldosterone levels increase from the first to third trimester; plasma renin is increased, and atrial natriuretic peptide is reduced suggesting the pregnant circulating system is under-filled compared to the non-pregnant state and that vasodilation precedes and predominates the increase in plasma volume. Blood pressure falls in the first trimester and reaches a nadir in the second trimester and rises to pre-pregnancy levels towards the end of the third trimester [12].
Red blood cell mass increases 20–30 % through pregnancy; this results in a mild reduction in haematocrit as the plasma volume rises more than red cell mass. This is most marked in the first half of the third trimester and results in reduced viscosity which further reduces systemic vascular resistance and afterload. These changes tend to resolve to pre-pregnancy values by 8 weeks postpartum. There is also an increase in concentration of coagulation factors [18] and a 6–22-fold increase in the risk of thromboembolism [19].
Glomerular filtration rate increases within the first month of conception, peaks before 20 weeks and declines at the end of the third trimester. This is due to increased renal blood flow secondary to increased cardiac output and renal vasodilation. This leads to a fall in creatinine by approximately a third.
60.4.3 Relevant Non-cardiovascular Physiological Changes
Oxygen consumption increases by 20 % at term, and minute ventilation increases by 50 % by term. This change in ventilation is predominantly mediated through an increase in tidal volume with little change in respiratory rate. The result of this mismatch in consumption and ventilation is an increase in pO2, a decrease in pCO2 and mild compensated respiratory alkalosis [20].
60.5 Evaluating the Pregnant Patient
60.5.1 History Taking
Some women develop symptoms during pregnancy, and it can be difficult to determine whether they are due to normal physiological changes or cardiovascular disease (Table 60.3).
Table 60.3
Symptoms and signs during healthy pregnancy and cardiac disease
Normal | Abnormal | |
|---|---|---|
Symptoms | Mild dyspnoea | Severe or progressive dyspnoea |
Fatigability | Paroxysmal nocturnal dyspnoea | |
Decreased exercise tolerance | Syncope with exertion | |
Palpitations | Palpitations | |
Signs | Mild pedal oedema | Severe peripheral oedema |
Full, sharp, collapsing pulse | Clubbing and cyanosis | |
Prominent LV impulse | Persistent neck vein distension | |
3rd heart sound | Cardiomegaly | |
Grade 1–2 ejection systolic murmur | 4th heart sound | |
Premature beats | Diastolic murmurs | |
Sustained arrhythmias |
60.5.2 Cardiovascular Examination: Points to Consider
Blood pressure measured after 20 weeks gestation should be assessed in the left lateral position or sitting up to avoid supine hypotensive syndrome where blood pressure falls due to reduced venous return to the heart due to uterine compression of the inferior vena cava. Murmurs develop in nearly all women during pregnancy. They are usually soft, mid-systolic and heard at the mid to upper left sternal border and are secondary to increased blood flow. A continuous bruit resulting from increased blood flow to the breasts, the ‘mammary soufflé’, can also be commonly heard.
60.5.3 Cardiac Investigations: Changes in the Pregnant Patient
ECG:
15–20° leftward shift in QRS axis, which can lead to small Q wave in III
Chest X-ray (CXR):
In normal pregnancy, the heart can be enlarged, with increased lung markings.
Typical radiation dose is 1.5 mGy to the mother and 0.5 mGy to the uterus of which there is no evidence of risk to the foetus.
Echocardiography:
As pregnancy progresses, the physical changes (elevated and splinted diaphragm) affect the echo windows, and subcostal windows in particular are more challenging in the third trimester.
Ejection fraction does not change significantly as the increase in CO is predominantly through heart rate and stroke volume changes due to increased chamber size.
LV end-diastolic volume increases by approximately 20 %, and left ventricular mass increases by approximately 50 %.
Valve function is unaltered, but the Doppler flow across valves is slightly increased (as a result of the increased stroke volume), but there is unlikely to be a significant gradient if there is no pre-existing stenosis.
MRI:
Limited data on the safety in pregnancy exists. It is recommended to be used only in the second and third trimester when diagnostic information cannot be gained from echocardiography.
Gadolinium crosses the placenta and, as the risks to the foetus are not known, should ideally be avoided in pregnancy.
Radiation Exposure
If a radiological investigation is indicated to exclude a significant pathology, it should be performed at any gestation, and chest X-rays confer a negligible radiation dose to the foetus at any gestation.
Childhood rates of leukaemia are not altered by doses below 5 mGy; at 10 mGy, there is a small increased risk of incidence of 1 case per 1,700 exposed individuals [23].
Congenital malformations are not increased at radiation doses below 100–200 mGy [23].
The radiation dose which a foetus is exposed to during a typical investigation: chest X-ray 0.01 mGy and CT pulmonary angiogram 0.005–0.1 mGy depending on gestation.
60.6 Delivery
60.6.1 Physiological Changes During Delivery
Catecholamine levels rise, blood loss to a variable degree is almost inevitable, and post-labour, a large volume of extravascular fluid is mobilised by the contracting uterus. This acts as an autotransfusion to attenuate the hypovolaemia caused by the blood loss. During uterine contractions, systolic blood pressure increases 15–25 %, and diastolic blood pressure increases 10–15 %. Cardiac output increases during stage 2 (delivery of the baby) and further still after delivery due to autotransfusion from the contracting uterus [24]. The majority of cardiovascular adaptations of pregnancy resolve by 6 weeks.
60.6.2 Potential Cardiovascular Problems During Delivery
There can be an increased tendency to arrhythmia due to increased sympathetic drive leading to tachyarrhythmias and Valsalva manoeuvres (e.g. bearing down and straining) leading to bradyarrhythmias. Blood loss and/or vasodilation due to regional anaesthesia or analgesia can lead to hypovolaemia and hypotension. Autotransfusion from the contracting uterus (postpartum), delivery drugs causing vasoconstriction (see drugs below) and aggressive fluid resuscitation can precipitate pulmonary oedema and acute heart failure.
60.6.3 Mode and Timing of Delivery
Spontaneous labour is appropriate for most women with cardiac conditions. Complex cardiac conditions or those requiring anticoagulation sometimes need elective delivery.
Vaginal delivery is associated with reduced blood loss and risk of infection compared to caesarean section and is usually recommended. After delivery, slow oxytocin infusion can prevent postpartum haemorrhage.
Caesarean section is usually indicated for obstetric rather than cardiologic reasons; however, it should be considered for those on oral anticoagulants in preterm labour, patients with Marfan syndrome and dilated aortic roots, those with aortic dissections and those with acute heart failure [10].
60.6.4 Epidurals and Regional Anaesthesia
These can be effective analgesia and reduce sympathetic drive. Effective regional anaesthesia also reduces the tendency and therefore the haemodynamic effects of pushing. However, it can reduce systemic vascular resistance and blood pressure which can be dangerous in those with obstructive lesions (such as HCM and aortic stenosis).
60.7 Cardiac Diseases
60.7.1 Coronary Heart Disease (CHD)
Maternal deaths due to CHD are increasing in the developed world. Pregnancy raises the risk of CHD by three- to fourfold. The risk of CHD is 30 times greater for a pregnant woman over 40 than for a pregnant woman under 20. Previously undiagnosed CHD usually presents in the third trimester. All pregnant women assessed with chest pain should have an ECG. Maternal mortality from acute MI can be up to 7 %. All those who died from CHD reported in the most recent CMACE report [4] had identifiable risk factors. Where stenting is required, bare-metal stents are recommended. The majority of literature on stenting during pregnancy reports the use of bare-metal stents, and the risk of drug-eluting stents is unknown; use of bare-metal stents also allows a potentially shorter duration of dual anti-platelet therapy which if continued is a significant risk factor for haemorrhage during delivery [10] (Chap. 21).
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


