, Desiree M. Younes2 and Eric J. Feldmann3
(1)
Department of Medicine and Radiology, Stony Brook University Hospital, Stony Brook, NY, USA
(2)
Department of Medicine, Stony Brook University Hospital, 100 Nicolls road, Stony Brook, NY, USA
(3)
Department of Radiology, Stony Brook University Hospital, Stony Brook, NY, USA
3.1 Introduction
Cross-sectional cardiac imaging is increasingly being used to characterize congenital heart defects (CHD) and their associated extracardiac abnormalities. Both cardiac CT (CCT) and cardiac magnetic resonance imaging (CMR) are helpful in the diagnosis and monitoring of medically managed or surgically corrected CHD in adults. Additionally, clinically silent defects in young or middle-aged adults are often incidentally found with cross-sectional imaging done for unrelated purposes. Unlike CMR, CCT provides only morphological data. However, due to the relative availability, heightened spatial resolution, and short acquisition time of CCT, it is an important tool in the evaluation of CHD [1–5].
3.2 Normal Cardiac Anatomy
Describing complex congenital defects has been simplified by the development of a “sequential segmental” approach to cardiac imaging [1, 2, 6–8]. This approach utilizes morphological criteria, rather than spatial orientation, to describe CHD.
Cardiac situs is first determined; this is defined by the relative relation of the atria. The left and right atria are differentiated from each other by their appendages: the left atrial (LA) appendage has a narrow opening and finger-like projections, while the right atrial (RA) appendage has a broader base and is triangular shaped. The RA also has a crista and pectinate muscles [9].
Subsequently, each of the three cardiac segments (atria, ventricle, and great arteries) is located. The morphological right ventricle (RV) is triangular; it contains a moderator band and coarse muscular trabeculations along the interventricular septum. The RV also has a muscular infundibulum separating the atrioventricular valve from its semilunar valve. The left ventricle (LV) is elliptical, has fine trabeculations, and is with fibrous continuity between the atrioventircular (AV) and semilunar valves. The tricuspid valve consists of three leaflets (septal anterior, superior, and inferior), which are supported by papillary muscles that arise from the trabeculations of the RV. The mitral valve has two leaflets, each supported by a papillary muscle group. The great arteries are defined by their branches, and the great veins are defined by the organs that they drain.
The next step in the sequential segmental approach in describing cardiac structure is to determine venoatrial, atrioventricular (AV), and ventriculoarterial (VA) connections. Normally, the RA is connected to the RV through the tricuspid valve and the LA to the LV through the mitral valve. This is termed concordance. Abnormal AV connections include discordant connections, atresia of the AV valve, and double inlet ventricle (defined as over 50 % of each AV valve overlying a single, dominant ventricle) [2, 9, 10]. Abnormal VA connections include discordance, atresia, double outlet ventricle, and the presence of a common arterial trunk (truncus arteriosus).
Associated cardiac anomalies should subsequently be identified. These include intracardiac anomalies, such as atrial and ventricular septal defects, as well as extracardiac anomalies, such as coarctation of the aorta, patent ductus arteriosus, and anomalous coronary arteries. The cardiac position within the chest and orientation of the apex should also be determined.
3.3 CHD in the Adult
Many types of CHD of simple to moderate complexity may not present until the second or third decade in life. In fact, at least 10 % of adults with CHD are not diagnosed until adulthood [11]. The incidental discovery of CHD on cross-sectional imaging done for noncardiac purposes is not uncommon [3]. Additionally, due to advances in pediatric cardiac care, 85 % of babies born with CHD are expected to reach adulthood [12]. Many of these patients do not establish cardiac follow-up when transitioning to adult care and may eventually present to regional cardiac centers [13]. Knowledge of the anatomy of CHD on cross-sectional imaging is important in the management of these patients.
3.4 Specific Congenital Heart Defects in the Adult
3.4.1 Atrial Septal Abnormalities
Atrial septal defects (ASD) are the most common congenital defect found in adults; they make up about 30 % of all lesions [14, 15]. The size of the shunt through the ASD increases with age, and by the age of 40, more than half of affected patients develop shortness of breath, fatigability, atrial arrhythmias, or, rarely, pulmonary hypertension [16–18].
Ostium secundum defects are the most common type of ASD, accounting for 75 % of defects. They are the result of a malformation of the fossa ovalis and are located centrally in the interatrial septum (IAS). Ostium primum defects, which account for 15 % of ASDs, occur at the site of the endocardial cushions in the lower part of the IAS. These defects are often associated with mitral and tricuspid regurgitation or ventricular septal defects. Sinus venosus defects make up 10 % of ASDs and result from the failure of the sinus venosus to incorporate into the right atrium. These defects occur at the junction of the superior or inferior vena cavae to the right atrium; the superior type is commonly associated with partially anomalous pulmonary venous return [2, 3, 16, 19, 20].
A patent foramen ovale (PFO) occurs when the septum primum and septum secundum, which normally fuse shortly after birth, remains patent [14]. This occurs in 20–34 % of people [21]. This defect is usually asymptomatic, although it can be associated with a range of illnesses such as migraines and sleep apnea. The most common presenting symptoms are related to paroxysmal emboli (stroke). An associated atrial septal aneurysm, defined as a redundancy in the IAS that results in a 10 mm protrusion of the septum beyond its plane [22], should be noted on cardiac imaging. The presence of an IAS aneurysm increases the risk of stroke and may affect the stability of percutaneous closure devices [16, 23].
Rarely, a defect in the roof of the coronary sinus may occur that leads to an abnormal communication between the LA and the coronary sinus. This results in intra-atrial left to right shunting similar to that seen with an ASD despite an intact IAS. An unroofed coronary sinus may be associated with a persistent left superior vena cava [20, 24].
Echocardiography is the imaging modality of choice in diagnosing ASDs as small defects may be missed on nongated cardiac CT [1]. However, cardiac CT can aid in assessing the size and location of a secundum ASD and its rims; this information is needed to determine the possibility of percutaneous, rather than surgical, closure [25, 26]. Sinus venosus ASDs, which are usually high in the IAS, may be missed on echocardiography and, along with anomalous pulmonary venous return, may require cross-sectional imaging for diagnosis.
Other atrial abnormalities, such as left atrial diverticulium and left atrial accessory appendages, are being diagnosed more often with the increased use of cardiac CT and likely represent normal variants [27–29]. LA diverticula have been reported in up to 36 % of patients undergoing cardiac CT; [29] 4.4 % of diverticula were noted on the IAS. Cardiac CT can also aid in the diagnosis of other benign structural heart defects. For example, lipomatous hypertrophy of the interatrial septum can be seen in up to 8 % of patients undergoing echocardiography and can lead to unnecessary invasive procedures [30–32]. On cardiac CT, this lesion appears as a mass of fat along the IAS that spares the fossa ovalis. The diagnosis of this lesion can be made with noninvasive cardiac imaging, rather than with invasive procedures [33].
3.4.2 Ventricular Septal Defects
Ventricular septal defect (VSD) is the most common cardiac congenital abnormality in children. With a prevalance of 0.08 %, it is the second most common cardiac congenital abnormality in adults (following ASD) [15]. Most VSDs close spontaneously by age 10; repair during childhood is considered only if associated with congestive heart failure (CHF) or pulmonary hypertension [19, 20]. VSDs that present in adulthood are usually small and without symptoms. If large enough, however, they can result in CHF or pulmonary hypertension [16].
The interventricular septum (IVS) is composed of an inlet, trabecular, and outlet components which surround a small, membranous septum at the base of the heart [9, 34]. Membranous VSDs make up 70 % of all VSDs [16]. These VSDs are bordered at least in part by fibrous continuity of an AV or arterial valve but may extend into the muscular septum. Muscular VSDs make up 20 % of all defects and are located in the trabecular septum, which extends from the membranous septum to the apex. The inlet portion of the IVS is located between the AV valves and their chordal attachments. Inlet VSDs are associated with other defects of the AV canal. Defects in the infundibular septum, which separates the right and left ventricular outflow tracts and is bordered superiorly by the semilunar valves, may result from malalignment and is more frequently seen with other CHDs such as tetralogy of Fallot.
3.4.3 Bicuspid Aortic Valve
Bicuspid aortic valve (BAV) is seen in 0.5–2 % of the population, more commonly in men [3, 9]. BAV results from abnormal development of the aortic valve commissures and fusion of the coronary cusps; 70–80 % of cases involve the right and left cusps. Symptoms are rare until the fourth or fifth decade of life, when the progressive valve calcification that results from turbulent blood flow leads to significant aortic stenosis (AS) or aortic insufficiency (AI) [3, 20].
CCT is useful in patients with BAV. Accurate measurements of the ascending aorta, which is frequently dilated in those with significant AS or AI, are needed prior to aortic valve replacement as concomitant aortic root replacement may need to be considered. Additionally, it allows for noninvasive assessment of coronary artery disease prior to open heart surgery. CCT can also asses for coarctation of the aorta, which is commonly associated with BAV.
3.4.4 Quadricuspid Aortic Valve
Quandricuspid aortic valve is rare congenital AV abnormality that leads to significant valvular disease in adulthood. It is found in up to 0.04 % of the general population but in 1 % of those undergoing surgery for “pure” AI [36, 37]. Fibrous thickening of the leaflets leads to incomplete coaptation and AI. Surgery is often required by the fifth or sixth decade of life [38].
3.4.5 Pulmonary Valve Abnormalities
Pulmonary stenosis makes up 10–12 % of cases of adult CHD. Valvular stenosis results from varying degrees of commissural fusion and is responsible for 90 % of cases [9, 19]. Adults with mild RV outflow tract obstruction often have no symptoms, but 20 % of those with moderate obstruction progress and develop evidence of right sided volume overload [39]. Intervention is indicated when peak pressure gradient surpasses 60 mmHg or when symptoms are present. Percutaneous balloon valvuloplasty is the treatment of choice [20].
Quadricuspid pulmonary valves are five times more common than quadricuspid aortic valves and develop from a partitioning of one of the three embryologic valve cushions during early valvulogenesis [40, 41]. When isolated, quadricuspid pulmonic valves are clinically silent and are diagnosed incidentally.
3.4.6 Ebstein’s Anomaly
Ebstein’s anomaly (EA) is rare, occurring in 1 out of 200,000 live births [42]. Failure of the septal and posterior leaflets of the tricuspid valve to delaminate from the underlying myocardium during fetal development results in apical displacement of the tricuspid annulus and “atrialization” of the right ventricle. This results in varying degrees of tricuspid regurgitation, and right atrial enlargement results [9]. An ASD is present in 50–94 % of patients due to atrial enlargement and gaping of a PFO [9, 42, 43].
Patients with only moderate tricuspid disease may not develop symptoms until early adulthood. These patients may develop arrhythmias, exercise intolerance, cyanosis, or right-sided heart failure. They are also at risk for paradoxical embolization through existing ASDs and brain abscesses [42, 44]. The diagnosis is usually made on echocardiography. Surgical repair of the tricuspid valve is indicated in patients that develop cyanosis, right-sided heart failure, decrease in functional capacity, and recurrent emboli. Relative indications for repair include asymptomatic cardiomegaly and recurrent atrial arrhythmias.
3.4.7 Patent Ductus Arteriosus
The ductus arteriosus is an arterial structure that connects the proximal left pulmonary artery to the descending aorta, just distal to the left subclavian artery. During fetal development, it serves to reroute deoxygenated blood from the RV to the aorta for oxygenation by the placenta. Normally, oxygen causes the duct to vasoconstriction and functionally close within 72 h of birth. In about 1 in 500 children, the duct fails to close; this is termed patent ductus arteriosus (PDA) [9, 14, 16, 45].
Small to moderate PDAs may remain silent or may present in adulthood as systemic arterial resistance rises and increases shunting to the pulmonary vasculature. Presenting features include pulmonary vascular congestion, atrial arrhythmias from LA enlargement, and endarteritis. The development of Eisenmenger’s syndrome may result in shunting of pulmonary arterial blood into the aorta distal to the left subclavian artery. This leads to cyanosis or clubbing of the lower extremities only, termed “differential cyanosis” [14, 45]. Clinical symptoms, volume overload, and endarteritis are indications for PDA closure.
Echocardiography can establish the diagnosis and assess for elevated pulmonary pressures and cardiac chamber enlargement. CCT is used to noninvasively characterize the size, morphology, and calcifications of the ductus. This information is needed for selecting the modality of closure (open versus transcatheter) and the size of the closure device [46].
3.4.8 Coarctation of the Aorta
Coarctation of the aorta is a narrowing of the aorta, usually between the left subclavian artery and the ductus arteriosus, due to a localized shelf in the aortic well or tubular hypoplasia of the aorta. Collateral vessels are often present, and some patients do not present until early adulthood. Hypertension in the upper extremities is common. Long-term complications are due to severe hypertension and include cerebral aneurysms and hemorrhage, hypertensive encephalopathy, aortic rupture, LV failure, and endocarditis [1, 2, 9].
Cross-sectional imaging establishes the diagnosis, degree and morphology of stenosis, and presence of collateral vessels [47, 48]. Repair is indicated when the gradient across the lesion is under 20 mmHg or when collateral vessels are present and often can be done with percutaneous stenting [20]. Postoperative monitoring with cross-sectional imaging is necessary to screen for complications such as restenosis and aneurysm formation [3, 9]. Metallic clips and stents cause streak artifact on MR; CT is often more useful in the postoperative patient.
3.4.9 Partial Anomalous Pulmonary Venous Return
Partial anomalous pulmonary venous return (PAPVR) is the drainage of one or more of the pulmonary veins directly or indirectly into the right atrium, seen in 0.3 % of adults [16]. In 79 % of cases presenting in adults, the left upper lobe of the lung drains to a left vertical vein, which drains into a dilated left brachiocephalic vein [49]. Only 17 % of adult cases involved drainage of the right upper lobe into the superior vena cava; patients with this anatomy, which is associated with sinus venosus ASD, may present at an earlier age. The pulmonary veins may also drain into the azygos vein or the coronary sinus. Pulmonary drainage into the inferior vena cava is termed scimitar syndrome and is associated with hypoplasia and abnormal arterial collateral supply of the right lung as well as dextrocardia [2, 9, 16].
Patients with PAPVR do not develop symptoms unless over 50 % of pulmonary return is anomalous or unless an ASD is present. View of the pulmonary veins may be limited on echocardiography; cardiac CT is helpful in establishing the diagnosis [50]. Associated abnormalities, such as enlargement of the right heart and pulmonary artery, can also be seen on CT [49]. Surgical intervention should be considered in cases of right ventricular volume overload [9].
3.4.10 Anomalous Systemic Venous Return
Persistence of the left superior vena cava (SVC), which results from failure of the left anterior cardinal vein to regress during fetal development, is seen in under 0.5 % of the general population and in 4 % of those with CHD [51]. When isolated, the left SVC usually drains into the coronary sinus. A right SVC is frequently present, and the two cavae may be connected by an anterior bridging vein [2]. When associated with CHD, it may drain into the LA [52]. Although this condition is not normally associated with symptoms, it may result in difficulties with placement of intravenous lines or intracardiac devices.
Other systemic venous anomalies in the thorax include abnormal drainage or dilation of the right SVC, abnormal course of the left brachiocephalic vein, and abnormal drainage of the inferior vena cava. These anomalies are rare and, when isolated, clinically silent [51].
3.4.11 Aortopulmonary Collaterals
Aortopulmonary collaterals (APCs) are remnants of embryonic ventral splanchnic arteries arising from the systemic arteries that provide blood to the pulmonary circulation [53, 54]. They are seen most commonly with cyanotic lesions, such as tetralogy of Fallot with pulmonary atresia and single ventricle heart disease, and the majority is found during infancy [55]. They can be newly diagnosed in adults and are associated with right lung hypoplasia. Their presence and the territory that they supply are important factors when considering shunt placement and other cardiac surgeries [2, 53].
3.4.12 Tetralogy of Fallot Repair
Tetralogy of Fallot (ToF) is the most common cyanotic CHD seen in adults. Malalignment of the septal components of the fetal heart leads to an overlying aorta, RV outflow tract (RVOT) obstruction, VSD, and RV hypertrophy. The degree of left-to-right shunting is determined by the severity of the RVOT obstruction. Most patients present shortly after birth, and without surgical correction, there is a near 90 % mortality by age 20 [9, 56, 57].
In the past, surgical repair was accomplished by creating conduits between the systemic and pulmonary artery (PA). Anastomosis can be created between the ascending aorta and right PA (Waterston operation), descending aorta to left PA (Potts operation), or subclavian artery to main PA (Blalock–Taussig operation). Currently, complete ToF repair is performed and consists of closure of the VSD and relieve of RVOT obstruction [56].
With surgery, the majority of patients live well into adulthood [58]. Postoperative complications include residual VSD or RVOT obstruction, pulmonary insufficiency that may lead to RV failure, or PA stenosis. CCT is useful for postoperative assessment of the PA and RV. CCT can also be used to evaluate cardiac defects associated with ToF, seen in 68 % of patients, such as right aortic arch and anomalous coronary arteries [1, 43, 59].
3.4.13 Congenital Absence of Pericardium
Congenital absence of pericardium is extremely rare, with only cases reported in the literature. The defect may be partial or complete, and it may be associated with other cardiac defects such as ASD or bicuspid aortic valve. Symptoms, if present, are most often sharp, paroxysmal chest pains. Life-threatening complications include torsion of the great vessels and herniation of portions of the heart through the defect [9, 60, 61].
Imaging reveals leftward shifting of the apex, right ventricular enlargement, and the presence of lung tissue between the aorta and left PA. MR can directly visualize the pericardium and confirm the presence and extent of the defect [62, 63]. Surgical reconstruction of the pericardium is considered in symptomatic patients.
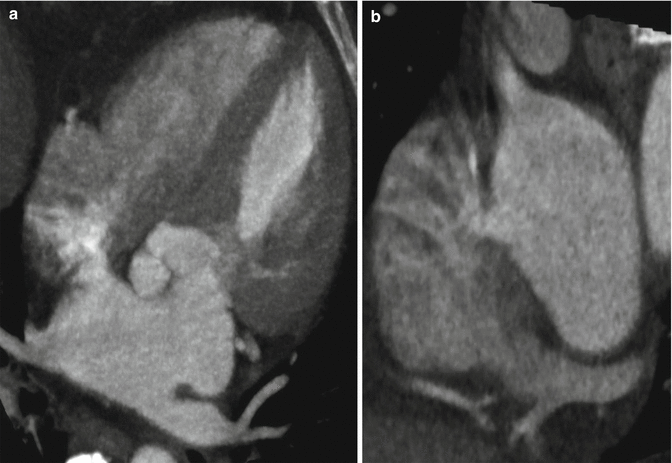
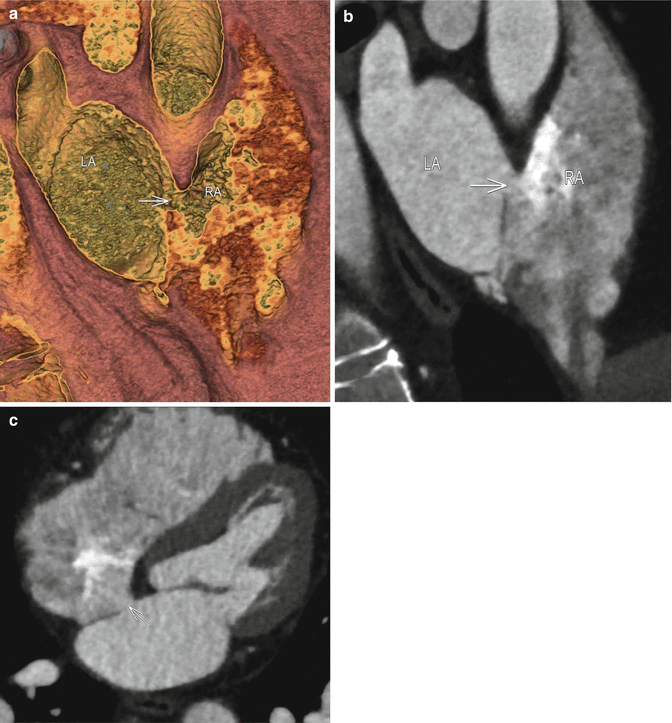
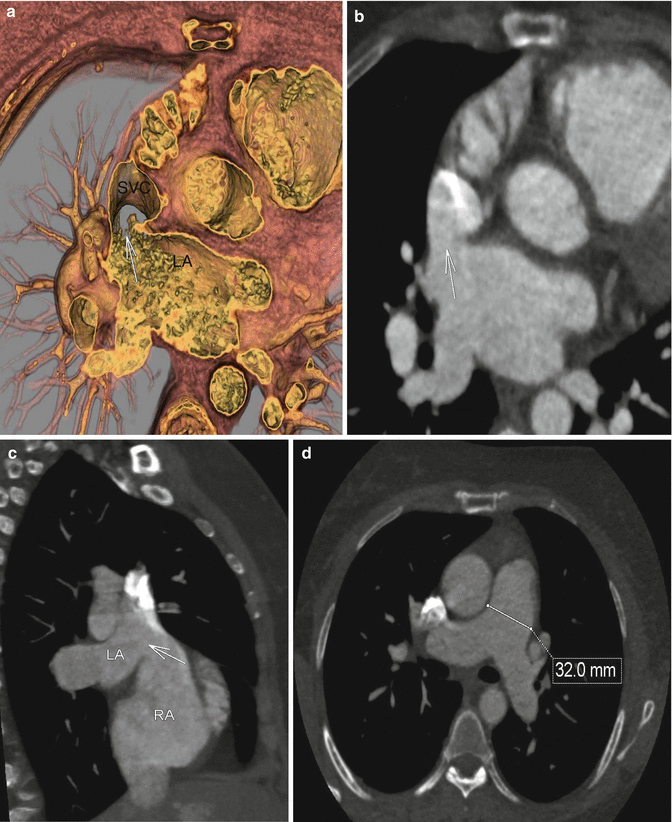
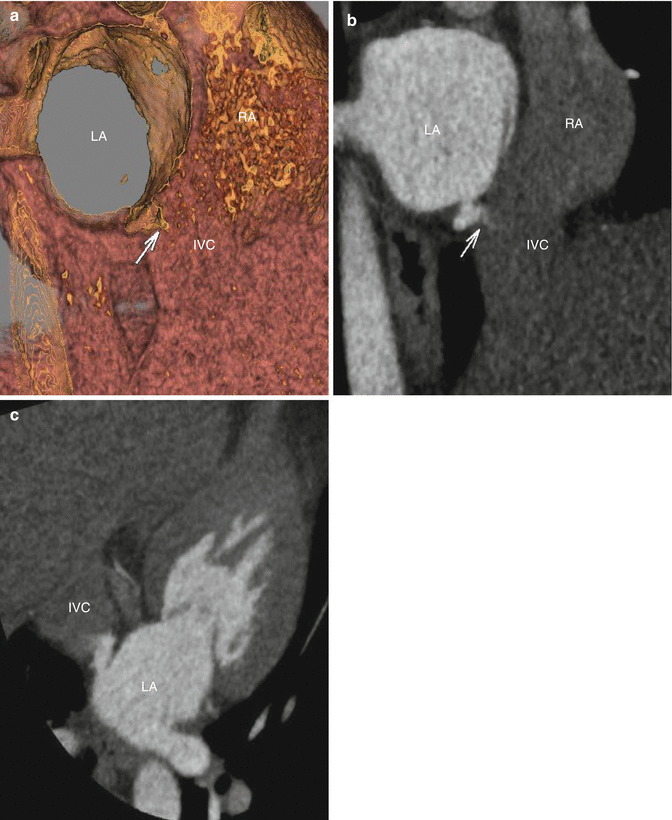
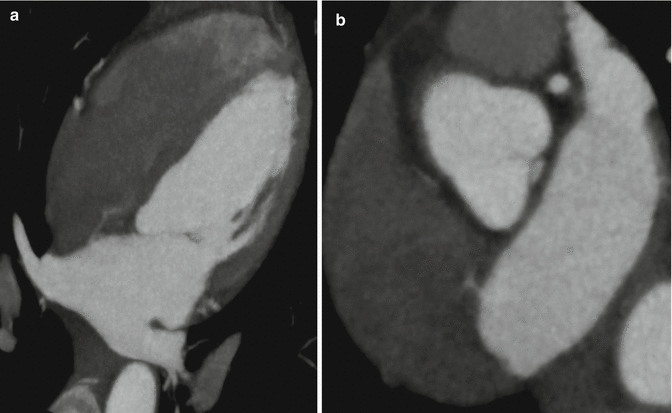
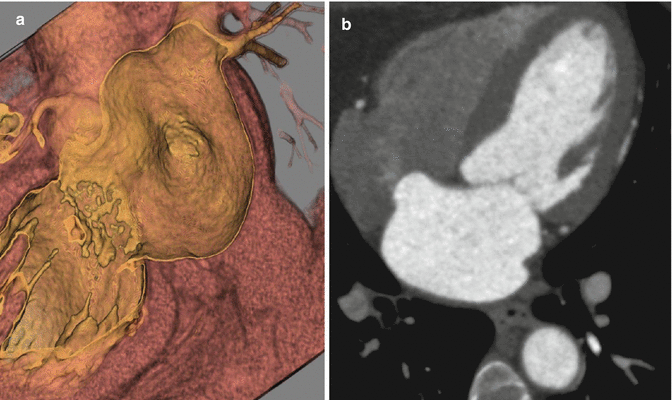





Fig. 3.1
(a) Axial near- four chamber maximum intensity projections at the level of the aortic root demonstrates left atrial contrast passing through a defect in the interatrial septum and into the right atrium. These findings are consistent with an atrial septal defect and should be corroborated with other projections and phases. In general, admixture artifact must be excluded, although it is typically not this robust. (b) Near short axis oblique multiplanar reformat at the level of the interatrial septum confirms left atrial contrast passing through a defect in the interatrial septum and into the right atrium. The location is most consistent with an ostium secundum defect

Fig. 3.2
(a) Near-short axis hollow volume rendered image at the level of the superior interatrial septum demonstrates a connection between with left atrium and right atrium, which is located far anteriorly (white arrow). (b) An oblique multiplanar reformat at the same level confirms this (arrow). Notice that the right atrium demonstrates prominent admixture artifact, as is typical. No prominent left to right contrast jet is present in this case, due to differences in contrast timing when compared to the first case. (c) Oblique multiplanar reformatted four chamber image at the superior aspect of the interatrial septum delineates the ostium primum defect (arrow). Note, how the contrast at the far left aspect of the right atrium is similar in attenuation to the left atrium, and is free of admixture artifact. LA left atrium, RA right atrium

Fig. 3.3
(a) Near axial projection hollow volume rendered image at the level of the superior vena cava/left atrium. Image demonstrates a connection between the far superior left atrium and the base of the superior vena cava at its entry to the right atrium (white arrow) consistent with a sinus venosus type atrial septal defect (superior vena cava type). (b) Oblique multiplanar reformat in the same projection demonstrates fairly homogenous contrast between the superior vena cava and the left atrium (arrow), with slight high attenuation admixture artifact from the continued injection of intravenous contrast. (c) Near sagittal oblique multiplanar reformat at the level of the right atrium demonstrates the left atrium connected to the superior vena cava at its insertion to the right atrium (white arrow). (d) Axial multiplanar reformat demonstrates an enlarged main pulmonary at 32 mm (normal up to 29 mm), which suggests pulmonary arterial hypertension in this patient with known left to right shunt physiology. SVC superior vena cava, LA left atrium, RA right atrium

Fig. 3.4
(a) Oblique hollow volume rendered image and (b) Maximum Intensity Projection at the level of Inferior vena cava (IVC)/left atrium (LA) demonstrate a connection between inferomedial wall of the left atrium and the base of inferior vena cava (IVC) (arrow). (c) Maximum intensity projection image in an oblique near four chamber at the level of inferior atrial septum demonstrates a contrast jet traversing the posterior aspect of the interatrial septum and entering the far cranial aspect of the inferior vena cava- diagnostic of an sinus venosus ASD (inferior vena cava subtype)

Fig. 3.5
(a) Near four-chamber maximum intensity projection at the left of the mid interatrial septum demonstrates a thin area of high attenuation contrast extending from the left atrium and passing through a small defect in the mid-interatrial septum, most consistent with a patent foramen ovale. This finding is easily seen when there is a prominent difference in attenuation between the left atrium and right atrium, as is typically seen with modest contrast administration (40–70 ml) and excellent right atrial contrast nulling from a saline chaser. (b) Near short axis multiplanar reformation at the level of the interatrial septum confirms a small defect in the mid-interatrial septum consistent with a patent foramen ovale

Fig. 3.6
(a) Volume rendered hollow image in a near two-chamber/long-axis projection of the left atrial lumen demonstrates a bulge of the interatrial septum away from the lumen- thus toward the right atrium. Findings are consistent with an atrial septal aneurysm. Typically these need to bulge 10 mm into the right atrium beyond the atrial septum, and are more accurately measured when there is continuous image acquisition throughout the cardiac cycle, as in echocardiography or MRI. (b) Oblique multiplanar reformation in a four chamber view demonstrates a prominent right-convex bulge of the interatrial septum, meeting criterion for an interatrial septal aneurysm, even on this static image in diastole. These are commonly associated with atrial septal defects, usually patent foramen ovale, which is suggested by the area of high attenuation contrast in the adjacent right atrium. This should be confirmed with other phases/projection and echocardiography
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


